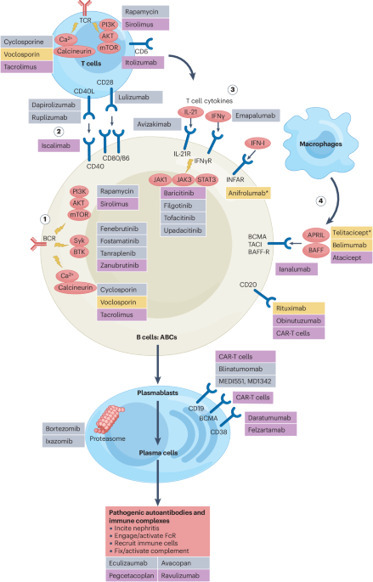
Celiac disease (CeD) is a common gastrointestinal disorder that can be diagnosed at any age. The disease is associated with intake of cereal gluten proteins, and the diagnostic scheme initially relied on elimination-provocation diets, typical of food intolerances. This has changed. Currently, diagnosis in pediatric patients can be made solely on the basis of the presence of high serum concentrations of autoantibodies (antibodies that recognize “self” antigens) to transglutaminase 2 (TG2, also known as TGM2 ), a cytosolic enzyme with broad tissue expression. Accumulating evidence indicates that these autoantibodies are formed as a result of an adaptive immune response to gluten and that interactions between gluten-specific T cells and TG2-specific B cells are important for development of CeD. No other human autoantibodies are better diagnostic markers for disease than TG2-specific antibodies. Without knowledge of disease dependence on dietary gluten, the presence of these autoantibodies would categorize CeD as an archetypical autoimmune disease rather than a food intolerance. Although autoimmune disorders are a collection of heterogeneous conditions, for which a single unifying mechanism is unlikely, some autoimmune diseases might share key pathogenic processes with CeD. Indeed, a provocative idea is that immune reactions to exogenous antigens can drive autoimmune diseases other than CeD (1). Environmental factors such as viral or bacterial infections have often been associated with development of autoimmunity. Yet, CeD is the only autoimmune disease for which pathogenic T cell epitopes originating from an exogenous antigen (gluten) have been identified. CeD shows strong association to certain genetic variants (allotypes) of major histocompatibility complex (MHC) class II molecules that mediate antigen presentation to CD4+ T cells. Hence, gluten-reactive CD4+ T cells are considered key pathogenic players in CeD. These CD4+ T cells reside in the gut lamina propria and release proinflammatory cytokines in response to gluten in the diet. As recently demonstrated in a mouse model of CeD, cytokines from CD4+ T cells control the action of cytotoxic intraepithelial lymphocytes (2). The result is release of cytotoxic molecules that kill epithelial cells and thus cause the typical destruction of the small intestinal tissue structure that is seen in CeD. The gluten epitopes that are recognized by the CD4+ T cells uniformly contain negatively charged glutamate residues that are important for binding to the CeD-associated MHC class II molecules. The glutamate residues are introduced into gluten peptides through deamidation, a posttranslational modification that is catalyzed by TG2. The dual role of TG2 in CeD as the target of autoantibodies and responsible for creating T cell epitopes can be explained in the context of T cell–B cell collaboration. TG2-specific B cells can take up TG2-gluten complexes through B cell receptor (BCR)–mediated endocytosis. Deamidated gluten peptides may then be presented to CD4+ T cells in a complex with MHC class II molecules on the surface of the B cells. The outcome is mutual activation of B cells and T cells, resulting in production of TG2-specific antibodies by the B cells and release of proinflammatory cytokines by the T cells (see the figure). The generation of autoantibodies in CeD implies breaking of B cell self-tolerance to TG2. However, a recent study in genetically modified mice expressing a CeD patient–derived, TG2-specific BCR suggested that there is no active induction of B cell tolerance to TG2 under normal conditions (3). The reason for the lack of tolerance induction is probably that TG2 is a cytosolic enzyme and that TG2-reactive B cells are therefore not exposed to extracellular antigen during their development. Hence, TG2-reactive naïve B cells are most likely continuously present both in CeD and in healthy individuals. In CeD, such B cells receive activation signals from gluten-reactive effector T cells. We hypothesize that once efficient T cell–B cell collaboration has been established, autoimmunity and tissue damage could ensue, in CeD and likely also in other autoimmune diseases. Although T cell–B cell interactions have been implicated in other autoimmune diseases, the well-characterized target epitopes in CeD offer distinct possibilities for studying pathogenic mechanisms. Hence, collaboration between TG2-specific B cells and gluten-specific T cells has been demonstrated both in vitro and in vivo (3). Gluten presentation by TG2-specific B cells was shown to depend on the TG2 epitope that is recgnized by the BCR (4). Thus, targeting of some TG2 epitopes allowed more efficient presentation of gluten on MHC class II than others, and antibody production against those epitopes correlated with the onset of clinical disease. Efficient T cell–B cell interactions therefore seem to be important for CeD development, and B cells are likely to be the main antigenpresenting cells (APCs) for pathogenic CD4+ T cells in inductive lymphoid structures. In addition, B-lineage cells may be involved in antigen presentation in nonlymphoid tissues. Plasma cells were recently identified as the main cell type presenting an immunodominant gluten epitope in gut biopsies of CeD patients (5). Plasma cells are terminally differentiated B cells whose main function is to secrete antibodies. However, plasma cells secreting immunoglobulin A (IgA) and IgM antibodies also express cell-surface immunoglobulins (6), which serve as functional BCRs (7), allowing receptor-mediated uptake of cognate antigen. Although the ability of plasma cells to stimulate CD4+ T cells has yet to be demonstrated, these observations suggest that they may act as APCs for tissue-resident CD4+ effector T cells in CeD. A role of B cells as the main APCs in CeD is supported by characteristics of gluten-reactive CD4+ T cells in blood and gut biopsies of CeD patients. These cells were recently described to have a distinct phenotype with features resembling those of T follicular helper (TFH) cells that are specialized for providing activation signals to B cells and that rely on B cell interactions for their differentiation (8). Thus, the CD4+ T cells expressed high amounts of the cytokine interleukin-21 and C-X-C motif chemokine ligand 13 (CXCL13), which are important for activating and attracting B cells. But, they lacked expression of the chemokine receptor CXCR5, which is required for homing to B cell follicles. A similar expression profile was observed in CD4+ T cells of patients with other autoimmune dieseases, including systemic lupus erythematosus (SLE) (8) and rheumatoid arthritis (9). The lack of CXCR5 expression suggests that inductive T cell–B cell interactions take place not in conventional germinal centers (GCs) in secondary lymphoid organs, but rather at extrafollicular sites such as the border between the T cell zone and the B cell follicle in lymph nodes or Peyer's patches of the gut. Extrafollicular activation of B cells in CeD is supported by the observation that TG2-specific antibodies rapidly disappear when patients start a gluten-free diet, indicating that GC-dependent long-lived plasma cells are not generated. Furthermore, these antibodies contain relatively few mutations, consistent with B cells being activated extrafollicularly rather than in GCs (6). Curiously, activated B cells in SLE patients were also found to lack CXCR5, consistent with a non–GC-dependent origin (10). Similarities between CeD and other autoimmune diseases may not be restricted to immune cell phenotypes and interactions. Common mechanisms could also guide the targeting of antigens. Similar to the deamidation of gluten in CeD, posttranslational modifications of antigens have been implicated in other autoimmune diseases. Yet, it remains to be established whether T cells specific to modified (self )-peptides are controlling tissue destruction in patients or if posttranslational modifications are a side effect of autoimmune reactions. Posttranslational modifications can potentially create neoepitopes that the immune system perceives as foreign, thereby facilitating escape of autoreactive cells from tolerance. The underlying mechanisms of neoepitope formation and their potential role in autoimmunity are poorly understood. Environmental factors such as smoking and viral infections are candidate triggers that may induce inflammatory tissue alterations, accompanied by dysregulation of posttranslational modifications and formation of neoantigens that could lead to autoimmunity. A prominent example of the connection between viral infections and autoimmunity is the association of Epstein-Barr virus (EBV) infection with development of multiple sclerosis (MS) (11)—a demyelinating autoimmune disease that affects the central nervous system. EBV persists in a latent state in memory B cells, which could serve as a permanent reservoir of viral antigens that can stimulate other immune cells. The disease is traditionally considered T cell mediated. Nevertheless, B cell depletion therapy with CD20-specific antibodies has a beneficial effect and limits relapses in MS, suggesting that B cells play an important role. If persistent viral antigens are important drivers of autoimmunity, a possible explanation for the clinical observations is that EBV-infected B cells are depleted by anti-CD20 therapy, thereby effectively removing the driving antigen (12). In this case, B cell depletion in MS would resemble the exclusion of gluten from the diet of CeD patients. Although intriguing, it has not been proven experimentally that viral antigens can drive autoimmune disease. Thus, the exact role of EBV in MS remains unclear, and it is not established whether EBV-specific T cells are pathogenic or if EBV-infected B cells in the central nervous system give rise to inflammation. Antibody production is typically considered the main function of B cells. However, B cells can play additional roles in regulation of immune reactions through secretion of cytokines or antigen presentation. Indeed, because anti-CD20 therapy does not deplete plasma cells, the clinical benefits of the treatment in MS strongly suggest that B cells have pathogenic involvement independent of antibody production. Circulating B cells in MS patients were shown to stimulate autoreactive, potentially pathogenic T cells that home to the brain (13). In addition, ablation of MHC class II expression specifically on B cells in mice resulted in amelioration of symptoms in experimental autoimmune encephalomyelitis, the primary mouse model of MS (14). Similar findings were also obtained in a mouse model of SLE (15), suggesting that antigen presentation by B cells to CD4+ T cells plays a key role in development of destructive immune reactions, at least in some autoimmune diseases. Dendritic cells are usually credited as the main APCs for T cells during an immune response. However, B cells are receiving increased attention as potent APCs in several autoimmune diseases. The main limitation to our understanding of pathogenic T cell–B cell interactions is the lack of well-defined target antigens in most autoimmune disorders. Characterization of T cell and B cell specificities will allow the study of disease-relevant immune cells that potentially can be targeted. Another major challenge is to understand why some people develop autoimmunity. Genetic predisposition is part of the answer, but environmental factors also play a role, possibly both by triggering and driving autoimmune reactions. Defining such factors is crucial for efficient treatment and prevention of autoimmune diseases. It is important to note that autoimmune disorders are a heterogeneous group of diseases with different manifestations and etiologies. Nevertheless, the mechanisms that are beginning to be unraveled in CeD could be relevant for other autoimmune conditions. http://www.sciencemag.org/about/science-licenses-journal-article-reuse This is an article distributed under the terms of the Science Journals Default License. References and Notes ↵ L. M. Sollid, B. Jabri, Nat. Rev. Immunol. 13, 294 (2013).OpenUrlCrossRefPubMed ↵ V. Abadie et al., Nature 578, 600 (2020).OpenUrl ↵ M. F. du Pré et al., J. Exp. Med. 217, e20190860 (2020).OpenUrl ↵ R. Iversen et al., Proc. Natl. Acad. Sci. U.S.A. 116, 15134 (2019). ↵ L. S. Høydahl et al., Gastroenterology 156, 1428 (2019).OpenUrl ↵ R. Di Niro et al., Nat. Med. 18, 441 (2012).OpenUrlCrossRefPubMed ↵ D. Pinto et al., Blood 121, 4110 (2013). ↵ A. Christophersen et al., Nat. Med. 25, 734 (2019).OpenUrlCrossRef ↵ D. A. Rao et al., Nature 542, 110 (2017).OpenUrlCrossRef ↵ S. A. Jenks et al., Immunity 49, 725 (2018).OpenUrl ↵ L. I. Levin, K. L. Munger, E. J. O'Reilly, K. I. Falk, A. Ascherio, Ann. Neurol. 67, 824 (2010). ↵ U. C. Meier et al., Clin. Exp. Immunol. 167, 1 (2012).OpenUrlCrossRefPubMed ↵ I. Jelcic et al., Cell 175, 85 (2018).OpenUrlCrossRef ↵ N. Molnarfi et al., J. Exp. Med. 210, 2921 (2013). ↵ J. R. Giles et al., J. Immunol. 195, 2571 (2015). Acknowledgments: The authors are supported by the University of Oslo World-leading research program on human immunology (WL-IMMUNOLOGY) and by grants from the South-Eastern Norway Regional Health Authority (project 2016113), the European Commission (project ERC-2010-Ad-268541), and Stiftelsen KG Jebsen (SKGJ-MED-017).

|
Scooped by
Gilbert C FAURE
onto AUTOIMMUNITY April 10, 2020 2:50 PM
|
No comment yet.
Sign up to comment





 Your new post is loading...
Your new post is loading...