Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
January 15, 2024 10:06 AM
|
Antinuclear Antibody Test Market Size, Share & Trends Analysis Report By Product (Reagents & Assay Kits, Systems, Software & Services), By Disease (Rheumatoid Arthritis, Systemic Lupus Erythematosus, Sjögren’s Syndrome, Scleroderma, Other Diseases), By Technique (ELISA, Immunofluorescence Assay, ...

|
Scooped by
Gilbert C FAURE
January 11, 2020 2:52 PM
|
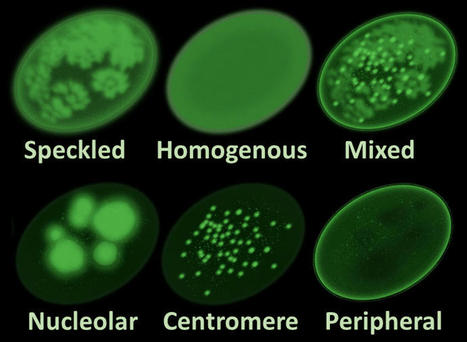
scleroderma and antibodies

|
Scooped by
Gilbert C FAURE
January 22, 2019 8:58 AM
|
The new blood test, sclero-smart™, measures levels of anti-vinculin, recently found to be elevated in patients with scleroderma and associated with gastrointestinal complications. This suggests a link between scleroderma and the gut microbiome, a finding that could open the door to discoveries of new microbiome-related therapeutic and diagnostic tools for scleroderma. Gemelli Biotech™, a specialty biotechnology company that develops diagnostics and therapeutics for the human microbiome, announced the commercial availability of sclero-smart™, an ELISA blood test that measures the level of an autoantibody called anti-vinculin in patients who have Systemic Sclerosis and Scleroderma (SSc). The clinical management of patients with SSc is typically very challenging, as the disease progression and organ involvement vary remarkably from individual to individual. There are a number of recognized autoantibody biomarkers that aid in understanding the progression of SSc in an individual. Anti-centromere, Anti-Scl-70 and Anti-RNA-pol-III are amongst the most common. The prevalence of these antibodies in the scleroderma population range from 10-30%. According to an abstract published recently by the American College of Rheumatology, up to 38% of people with scleroderma tested positive for elevated anti-vinculin antibodies, making anti-vinculin more prevalent in the scleroderma population than any other marker currently used for testing. Moreover, scleroderma subjects with elevated anti-vinculin levels had greater gastrointestinal (GI) symptom severity, an association no other marker provides. SSc commonly affects the GI tract, with symptoms including intestinal dysmotility, vomiting, bloating, anorexia, severe abdominal pain, small intestinal bacterial overgrowth (SIBO) and severe malnutrition. No other marker currently available is reliable in predicting the involvement of the GI system. Given the known link between anti-vinculin and intestinal neuropathy, anti-vinculin has substantial potential to serve as a marker for GI complications in scleroderma patients. According to another abstract published by the American College of Rheumatology, anti-vinculin was also associated with pulmonary hypertension. Few markers have an association with this serious vascular complication. Therefore, the measurement of anti-vinculin can be an important test to help rheumatologists identify the risk of GI or vascular complications in scleroderma patients. sclero-smart™ is the only commercially available test that measures anti-vinculin and has been developed through a partnership with the Medically Associated Science and Technology program at Cedars-Sinai. Dr. Mark Pimentel, Executive Director of the MAST program, said We know from a substantial volume of research that bacterial infection in the gut can cause an autoimmune response that involves the abnormal production of anti-vinculin. The connection between anti-vinculin and scleroderma means that the gut microbiome could help us answer many of the questions we still have about diagnosing and treating scleroderma. Matt Mitcho, CEO of Gemelli, added, Given how common the antibody appears to be in the patient population, this test could provide us with a more detailed understanding of the disease’s underlying causes. As a potential catalyst for such a breakthrough, this test is an important development for helping patients with scleroderma.

|
Scooped by
Gilbert C FAURE
September 23, 2018 2:58 PM
|
We examined the association of anti-RNPC3 antibodies in systemic sclerosis (scleroderma or SSc) patients with selected gastrointestinal (GI) tract complications...

|
Scooped by
Gilbert C FAURE
January 5, 2018 1:07 PM
|
A chronic autoimmune disease of the skin, called scleroderma, is not contagious or infections and often not harmful. However, when this connective tissue d

|
Scooped by
Gilbert C FAURE
February 21, 2017 4:44 AM
|
Introduction: Prior studies have demonstrated an increased risk of cancer‐associated scleroderma in patients with RNA polymerase III (POL) autoantibodies and in patients negative for anti‐centromer

|
Scooped by
Gilbert C FAURE
November 3, 2016 7:36 AM
|
The prognosis for patients diagnosed with scleroderma - an autoimmune disease characterized by fibrosis of the skin - is not typically a rosy one.

|
Scooped by
Gilbert C FAURE
December 31, 2015 2:35 AM
|
Objective: Our aim was to examine the association between anti-interferon inducible protein 16 (IFI16) antibodies and clinical features of scleroderma.

|
Scooped by
Gilbert C FAURE
November 13, 2015 3:48 AM
|
Here a team of researchers reported that systemic sclerosis is associated with alterations in sub-populations of B cells.

|
Scooped by
Gilbert C FAURE
May 29, 2015 2:11 PM
|
Arthritis Rheumatol. 2015 Apr;67(4):1053-61. doi: 10.1002/art.39022. Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't

|
Scooped by
Gilbert C FAURE
January 12, 2015 1:17 PM
|
Science. 2014 Jan 10;343(6167):152-7. doi: 10.1126/science.1246886. Epub 2013 Dec 5. Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't

|
Scooped by
Gilbert C FAURE
April 28, 2014 1:37 PM
|
Researchers Identify Biomarker in Helping Curtail PAH Caused By Scleroderma ...

|
Scooped by
Gilbert C FAURE
February 14, 2014 2:06 PM
|
Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma 7thSpace Interactive (press release) IntroductionWe assessed the profile and frequency of malignancy subtypes in a large single centre UK cohort for patients with...
|

|
Scooped by
Gilbert C FAURE
December 28, 2020 12:35 PM
|
Osteopontin Linked to Progressive Lung Scarring in Scleroderma Patients...

|
Scooped by
Gilbert C FAURE
April 2, 2019 9:33 AM
|
The Research Report on Antinuclear Antibody Test Market provides a detailed overview of the major drivers, restraints, challenges, opportunities, current market trends, and strategies impacting the ANA testing market, along with revenue estimates & forecast and market share analysis. Major Insights Covered in research Study: It provides valuable information on the products, techniques, and diseases in the ANA testing market. Details on regional markets for these segments are also presented in this report. Also, leading players are profiled to study their product offerings and understand the strategies undertaken by them to be competitive in this Antinuclear Antibody Test Market. Download PDF Brochure (Antinuclear Antibody Test Market): https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=218189007 Market Segment by Diseases for Antinuclear Antibody Test Market; Rheumatoid Arthritis Systemic Lupus Erythematosus Sjögren’s Syndrome Scleroderma Other Diseases Market Segment by Techniques for Antinuclear Antibody Test Market; Based on techniques, the Antinuclear Antibody Test Market is segmented into enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay (IFA), and multiplex assay. The ELISA segment is expected to account for the largest share of this market due to the expanding applications of ANA in autoimmune disease testing and therapeutic drug level monitoring. Speak to Our Subject Expert:https://www.marketsandmarkets.com/speaktoanalystNew.asp?id=218189007 Geographical Growth Insights for Antinuclear Antibody Test Market; North America is the largest regional segment in the ANA testing market, followed by Europe, Asia, and the Rest of the World (RoW). Growth in the North American market is primarily driven by the high incidence of autoimmune diseases, growing population and healthcare spending, and growth in the number of individuals covered under medical insurance in the U.S. Major Key Players Impacting the Global Market for Antinuclear Antibody Test; The major players in the antinuclear antibody test market are Alere Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), ERBA Diagnostics, Inc. (U.S.), Trinity Biotech plc (Ireland), Thermo Fisher Scientific, Inc. (U.S.), Antibodies, Inc. (U.S.), EUROIMMUN AG (Germany), Immuno Concepts (U.S.), Inova Diagnostics (U.S.), and Zeus Scientific, Inc. (U.S.). Request Research Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=218189007 Thermo Fisher Scientific was the leading player in the global ANA testing market. The company has a strong presence in North America, Europe, Asia, and Latin America. It focuses on expanding its presence in the ANA testing market by strategically investing in research and development activities, which enables it to launch new products in the Antinuclear Antibody Test Market. Tags:Antinuclear Antibody Test · Antinuclear Antibody Test industry · Antinuclear Antibody Test industry Analysis · Antinuclear Antibody Test Market · Antinuclear Antibody Test Market forecast · Antinuclear Antibody Test Market growth · Antinuclear Antibody Test Market key players · Antinuclear Antibody Test Market Research · Antinuclear Antibody Test Market Share · Antinuclear Antibody Test Market size · Antinuclear Antibody Test SALES

|
Scooped by
Gilbert C FAURE
December 17, 2018 2:20 PM
|
Summary Systemic sclerosis (SSc) is an idiopathic systemic autoimmune disease. It is characterized by a triad of hallmarks: immune dysfunction, fibrosis and vasculopathy. Immune dysfunction in SSc is characterized by the activation and recruitment of immune cells and the production of autoantibodies and cytokines. How immune abnormalities link the fibrosis and vasculopathy in SSc is poorly understood. A plethora of immune cell types are implicated in the immunopathogenesis of SSc, including T cells, B cells, dendritic cells, mast cells and macrophages. How these different cell types interact to contribute to SSc is complicated, and can involve cell‐to‐cell interactions and communication via cytokines, including transforming growth factor (TGF)‐β, interleukin (IL)‐6 and IL‐4. We will attempt to review significant and recent research demonstrating the importance of immune cell regulation in the immunopathogenesis of SSc with a particular focus on fibrosis. Introduction Systemic sclerosis (SSc) is an idiopathic systemic autoimmune disease. The pathogenesis of SSc is mostly unknown. Immunological abnormality, which includes autoimmunity and infiltration and activation of immune cells, is one of three key features of the disease. The other two hallmarks of SSc are fibrosis and vasculopathy. How autoimmunity is linked to fibrosis and vascular damage remains poorly understood, with no single hypothesis or mechanism to explain the links. It is therefore likely that a combination of mechanisms is involved in the pathogenesis of SSc, and picking apart these mechanisms to establish how the triad of fibrosis, vasculopathy and immune cell activation progress in SSc is a mammoth task. Many studies have linked immune abnormality as a cause of or at least a contributor to fibrosis in SSc, while fibrosis may also contribute to immune cell activation. Fibrosis in SSc occurs mainly in the skin but may progress to visceral organs, including heart and lungs. Fibrosis is the result of activation of fibroblasts and excessive extracellular matrix (ECM) deposition, both of which are hallmarks of SSc. As well as fibrosis, immune abnormalities are also linked to vasculopathy in SSc, with most research implicating vascular damage as an activator of immune cells. However, this review will focus mainly on the role of immunological abnormalities in the development of fibrosis in SSc; we recommend the paper by Asano and Sato for a comprehensive review of vasculopathy in SSc 1. Multiple different cell types of the immune system have been implicated in SSc, including T cells, B cells, dendritic cells, mast cells and macrophages. It appears that both innate and adaptive immunity is critical in the disease. We will attempt to review the old and emerging literature to examine the potential roles of these individual cell types in the immunopathogenesis of SSc. First, we will summarize major cytokines implicated in the immunopathogenesis of SSc, because these major players reappear in many studies, linking immune cell activation with other phenotypes in SSc, especially fibrosis. Cytokines in SSc Many cytokines are elevated in SSc, but interleukin (IL)‐4, transforming growth factor (TGF)‐β and IL‐6 are considered to be the major fibrogenic cytokines, or are at least the most studied. Genetic deletion of both IL‐4 and TGF‐β prevents skin fibrosis in a mouse model of SSc 2. Many other in‐vitro studies support the idea that IL‐4 promotes fibrosis through its ability to enhance the production of collagen 3, 4 and other ECM proteins 5, 6 while antibodies against IL‐4 prevent dermal fibrosis in the tight skin (Tsk) mouse model 7 targeted deletion of IL‐4 receptor in the Tsk mouse also reduces fibrosis 2. Skin and lung in SSc have high levels of IL‐4 8 and increased levels of IL‐4 in the blood are a common feature in patients with SSc 9-11 suggesting systemic release. TGF‐β is a well‐known potent inducer of fibrosis, with TGF‐β‐stimulated fibroblasts resembling those from SSc patients 12. Activation of the TGF‐β receptor following the binding of TGF‐β results in the phosphorylation and activation of SMAD proteins in the cytoplasm 13. TGF‐β also activates the three mitogen‐activated protein kinase (MAPK) signalling branches, c‐Jun N‐terminal kinase (JNK), p38 and extracellular signal‐regulated kinases 1 and 2 (ERK1 and 2) 12 all of which can promote inflammatory signalling. TGF‐β‐induced collagen production from both healthy and SSc dermal fibroblasts was found to be dependent on p38 14. JNK activation has also been implicated in fibrosis 15. However, in one study ERK activation inhibited skin fibroblast collagens I and III production while, p38 activation up‐regulated collagen I 16. IL‐6 is a classic proinflammatory cytokine and is also considered to be an important protein in the immunopathogenesis of SSc. For example, IL‐6 levels are increased in SSc patient sera 9 and skin 17. IL‐6 levels also correlate with SSc disease severity 18. A mouse model with development of autoimmune disease with SSc‐like skin thickening and lung fibrosis was found to be mediated by IL‐6 signalling 19. Bleomycin‐induced lung inflammation with collagen deposition was significantly attenuated in IL‐6‐deficient mice 20. IL‐6 signalling through trans‐signalling appears to be important, and we found that IL‐6 and the soluble form of the IL‐6 receptor are necessary for collagen production 21. We further showed in the same study that this was critical, dependent on the downstream signalling molecule signal transducer and activator of transcription (STAT)‐3. A crucial early step hypothesized to trigger the immune abnormalities and fibrosis in SSc is vasculopathy, including the damage and apoptosis of endothelial cells, resulting in the release of internal damage‐associated molecular patterns (DAMPs), which go on to activate and recruit immune cells 22. IL‐6 was found to mediate endothelial activation and apoptosis caused by the serum of patients with SSc 23, suggesting that it may play a major role in the very early stages of SSc. However, IL‐6 was found to be up‐regulated at the late stage of the disease using immunohistological analysis of skin biopsies from SSc patients 17. In both IL‐6 knock‐out (KO) mice and mice exposed to an IL‐6 blocking antibody, bleomycin‐induced dermal fibrosis was greatly induced by supressing fibroblast activation 24. The anti‐IL‐6 receptor antibody tocilizumab has had promising results with softening of the skin in two patients with SSc in one study 25 while a Phase II trial provided SSc patients with improvement in fibrosis of the skin 26 although statistically this was not significant. Thus, IL‐6 antibody therapy could be the first biological licensed for SSc. T cells T cells have been identified early in SSc progression before any evidence of fibrosis 27. SSc skin has a greater propensity to recruit/adhere T cells compared to healthy controls because of a greater expression of intercellular adhesion molecule (ICAM‐1), which is a ligand for the lymphocyte function‐associated antigen 1 (LFA1) receptor found on the surface of lymphocytes such as T cells 28. T cells from SSc skin biopsies have increased expression of the early T cell activation marker CD69 29. TGF‐β, which is elevated in SSc, was also found to be important for the recruitment of T cells to the skin in an SSc mouse model 30. A recent paper demonstrated that abatacept, which is an antibody that interferes with T cell activation, reduced fibrosis in not one, but two animal models of fibrosis 31. This was associated with reduced T cell activation and reduced levels of IL‐6, which may be mediated by blockade of cross‐talk between T cells and antigen‐presenting cells such as monocytes. Abatacept works by blocking the interaction of CD80/86 with cytotoxic T lymphocyte antigen (CTLA)‐4 on T cells, which is required for co‐stimulatory activation of T cells alongside major histocompatibility complex (MHC) and antigen presentation from antigen‐presenting cells such as dendritic cells, macrophages and B cells 32 Abatacept was initiated in three patients with morphea, and all three showed regression of the modified Rodnan skin score 33. T cells can be further classified into diverse subsets characterized by their distinct function, activators and the cytokines they subsequently release. Some of these T cell types have been found to play a role in SSc, which has been reviewed in more detail 34. However, many studies have identified the T helper type 2 (Th2) subtype as playing the largest role in SSc. Th2 cells are a major IL‐4‐producing cell type, but also secrete IL‐5, IL‐6, IL‐10 and IL‐13. Depletion of CD3+ T cells in a bleomycin mouse model reduced both fibrosis and IL‐4 secretion 35. As described above, IL‐4 can directly induce fibroblasts to promote fibrosis, but in addition IL‐4 can induce TGF‐β production in fibroblasts 36 and macrophages 37, which then leads to further signalling to fibroblasts to produce more collagen. Therefore, it is very likely that T cells have a major role to play in the onset of fibrosis in SSc through activation of macrophages and fibroblasts. An unusual population of CD4+CD8+ double‐positive T cells has been described in the skin that has very high levels of IL‐4 38. Gross et al. showed almost 30 years ago that IL‐4 is required to induce naive T cells to produce further IL‐4 39. If IL‐4 is required for T cells to produce IL‐4, this leads to the question: ‘what is the initial source of IL‐4 in SSc?’. This question remains unanswered, but potentially implicates other immune cells in the onset or progression of SSc. IL‐13 is produced predominantly by activated Th2 cells 34. IL‐13 was established as a major profibrotic agent in a model of hepatic fibrosis 40. IL‐13 inhibited IL‐1β‐induced matrix metalloproteinase (MMP)‐1 and MMP‐3 production and enhanced tissue inhibitor of metalloproteinase (TIMP)‐1 and collagen generation in fibroblasts 41. In the bleomycin model of SSc pulmonary fibrosis IL‐13 levels increased with pathogenesis, while neutralization of IL‐13 attenuated bleomycin‐induced pulmonary fibrosis 42. The potent fibrosis‐inducer, TGF‐β, may also contribute to an increase in Th2‐originating IL‐13 because TGF‐β up‐regulates GATA binding protein 3 (GATA‐3) expression in the T cells of patients with SSc, resulting in an increase in IL‐13 synthesis 43. As well as CD4+CD8+ double‐positive T cells, CD8+ single‐positive cells have been described that produce exuberant levels of IL‐13 44. These cells appear to be memory CD8+ T cells 45. We have shown that IL‐13 is directly profibrotic and is STAT‐6‐dependent 46. Thus, IL‐13 produced from T cells probably contributes to fibrosis in SSc and a monoclonal IL‐13 clinical‐grade antibody exists. Very recently, IL‐13 was shown to decrease MMP‐1 expression in both healthy and SSc fibroblasts and therefore may have an anti‐fibrotic role 47. However, this was only demonstrated in tumour necrosis factor (TNF)‐α‐induced fibroblasts, thus the effect of IL‐13 may be specific to the mechanism with which TNF‐α induces MMP‐1 expression. Another cytokine, IL‐17, prevalent in the serum of SSc patients in some studies, also implicates T cells 48. Th17 cells, which are characterized by their production of IL‐17A, IL‐17F, IL‐21 and IL‐22, are elevated in SSc skin compared to healthy controls 49, 50. Thus, Th17 cells and the IL‐17 they produce may play an important role in SSc. However, the roles of Th17 and IL‐17 in SSc are controversial, and have been recently reviewed in detail 51. Importantly, some studies have not detected differences in IL‐17 levels between SSc patients and healthy controls 52, 53 while IL‐17 has been shown to both increase 50 and decrease 54, 55 collagen production. The reasons for the differences in these studies between pro‐ or anti‐fibrotic effects of IL‐17 are not clear, and may reflect the source of the recombinant protein. A reduction in regulatory T cells has been recorded in the skin lesions of patients with SSc 56 suggesting that there is compromised capacity to regulate immune responses in SSc. A recent study identified an imbalance of regulatory T cells and Th17 cells, with a decrease in the former and an increase in the latter in the peripheral blood of SSc patients compared to healthy controls 57. In addition, a more recent study has confirmed that Th2 and Th17 cells are found in higher frequencies in SSc patients, and this was found to positively correlate with IL‐35 levels, although a causal link has not been established 58. Further evidence of disruption to T cell homeostasis comes from a recent study showing that SSc patients displaying severe peripheral vascular complications have an expansion of the recently discovered angiogenic T cells 59. This expansion in angiogenic T cells may also demonstrate a link between immune cell activation and vasculopathy in SSc. Macrophages More than 30 years ago circulating monocytes were found to be strongly activated in patients with SSc 60. Since then, many studies have found evidence of monocyte/macrophage activation in SSc. Higher numbers of macrophages have been observed in skin from SSc patients 27, 61 while cells positive for CD163, a putative marker for M2 macrophages 62, have been found to be increased in the serum of SSc patients 61, 63-65. M2 macrophages are therefore prominent in SSc, with a very recent study involving more than 200 SSc patients, not only confirming this but also demonstrating that soluble CD163 (sCD163) is significantly elevated in the serum of SSc patients and therefore may be of use as a biomarker 66. Microarray gene expression data have also supported a role for monocytes/macrophages in the immunopathogenesis of SSc, with studies demonstrating that peripheral blood mononuclear cells (PBMCs) from SSc patients have an increased expression of genes associated with monocyte/macrophages 67-69. Such large‐scale genomic studies have identified multiple innate immune regulators in SSc. Macrophages are also a major source of TGF‐β 70, which is a potent inducer of fibrosis 71. Many studies have implicated macrophages in the initiation or progression of fibrosis in SSc. Microarray data from SSc lung tissue confirm what has been observed in the blood with markers of macrophage activation 72 and emigration, which correlate with progressive lung fibrosis 73. In another recent study, a novel multi‐network approach to compare gene expression profiles has identified a gene expression signature indicative of profibrotic M2 macrophages in SSc tissues 74. Interestingly, the profibrotic macrophage gene expression profile differed between skin and lung, suggesting that although a role for macrophages in the immunopathogenesis of fibrosis in SSc seems likely in both skin and lung, there may still be subtle differences in what those roles are. Recently nintedanib, an inhibitor of the receptor tyrosine kinase platelet‐derived growth factor receptor, fibroblast growth factor receptor and vascular endothelial growth factor receptor, blocked myofibroblast differentiation and subsequent fibrosis in a mouse model of SSc. Interestingly, these nintedanib‐mediated anti‐fibrotic effects were associated with reduced numbers of M2 macrophages 75. Many chemokines which both recruit and can be primarily secreted by macrophages are up‐regulated in SSc skin 76-79 which is not surprising, given that infiltration of macrophages in SSc skin has been known since the early 1990s 80. However, the chemokine (C‐C motif) ligand 19 (CCL19) was up‐regulated in SSc skin and co‐localized with CD163‐positive macrophages, suggesting that it has a role in the recruitment of macrophages 76. The authors of the study also suggest that macrophages are the source of the CCL19, and in‐vivo experiments demonstrate that Toll‐like receptor (TLR)‐3, ‐4 or ‐9 activation is required for CCL19 expression in monocytes. TLR activation is considered a major event in the immunopathogenesis of SSc 81, 82. Cell types which highly express TLRs such as macrophages are thought to play a major role in SSc. However, many different cell types express TLRs, and thus TLR‐mediated signalling in the immunopathogenesis of SSc may not be limited to macrophages. It is possible that TLR activation in any cell type expressing TLRs is also a source of CCL19, and therefore a potential cause of macrophage recruitment in SSc. Indeed, Mathes et al. also observed that CCL19 expression was up‐regulated from TLR activation in T cells and other cell types from PBMCs, but monocytes gave the most robust increase 76. We have found that macrophages that respond to the TLR‐8 stimulus single‐stranded RNA in SSc result in up‐regulation of TIMP‐1 that is functional and leads to increased collagen deposition 83. Interestingly, these monocytes seemed to be perivascular. IL‐6 appears to play an important part in the role of macrophages in SSc because inhibition of phosphodiesterase‐4, which blocks M2 differentiation, also inhibits IL‐6 production, fibroblast activation and fibrosis in an SSc mouse model 84. IL‐6 may also be an important activator of M2 macrophages, as indicated by IL‐6 receptor blockage by tocilizumab 85 causing down‐regulation of genes associated with M2 macrophages in SSc skin. Oncostatin M is another IL‐6‐like cytokine that appears to be involved in fibrosis, as cells treated with OSM induced ECM accumulation in fibroblasts 86. SSc patients with higher expression of the M2 macrophage marker, MRC1, were also found to have elevated levels of IL‐13 in their plasma 69 suggesting that macrophage activation in SSc results in production of cytokines from macrophages themselves or indirectly through interaction with other immune cells, because macrophages can both secrete IL‐13 87 and, through being antigen‐presenting cells, can activate T cells which could lead to IL‐13 production. There have been more than 30 years of research implicating macrophages in the immunopathogenesis of SSc, with various cytokines produced by macrophages and other immune cells, internal molecules from damaged endothelial cells and chemokines all contributing to the recruitment and activation of macrophages, resulting in a profibrotic environment (Fig. 1). However, how macrophages are recruited to and activated in the tissues of SSc patients is not fully understood. What role is played by macrophages in the progression of fibrosis is also not fully known, but activation of TLRs and the subsequent profibrotic signalling remains an obvious choice. Targeting of TLRs may be a therapeutic option. B cells B cells are heavily implicated in the immunopathogenesis of SSc. B cells are known inducers of fibrosis generally and in SSc 88 and mounting evidence suggests that they are modified and activated in patients with SSc. SSc patients have abnormalities of B cell homeostasis in the blood, which includes an expansion of naive B cells and activated but diminished memory B cells 89. The cytokine B cell activating factor (BAFF), which belongs to the TNF ligand family, is a potent activator of B cells 90. Not only are BAFF levels elevated in SSc; levels also correlate with disease severity, while B cells isolated from SSc patients produce more IL‐6 when exposed to BAFF 91. A BAFF antagonist inhibited IL‐6 and IL‐10 expression in the skin of the Tsk mouse model of SSc, while stimulation of B cells with BAFF greatly increased IL‐6 92. IL‐6 can direct the differentiation of T cells into IL‐4‐producing Th2 cells 93, therefore B cell‐secreted IL‐6 could contribute to the Th2 phenotype detected in SSc. As well as being involved in IL‐6 cytokine production, B cells are also suggested to play an important role in fibrosis. Co‐cultures of B cells and fibroblasts from SSc patients induced a fibrotic response, including collagen, TGF‐β1 and IL‐6 secretion. Exposure of co‐cultures to BAFF (and anti‐IgM) further increased secretion of the profibrotic compounds 88. The majority of B cells are IL‐6‐producing, termed B effector cells (Beffs), and have a proinflammatory and autoimmunity role. However, a small subset of B cells termed regulatory B cells (Bregs) are potent negative regulators of inflammation and autoimmunity, in part through their ability to express IL‐10 94. These Bregs therefore have the potential to inhibit diseases with immune abnormalities such as SSc. Indeed, Bregs were able to inhibit the initiation of a mouse model of multiple sclerosis and depletion of Bregs increased symptom severity 95. Interestingly, the frequency of blood Bregs was found to be significantly lower in SSc patients compared to healthy controls 96 while in a very recent study by Matsushita et al., Bregs were found to have a similar role in a mouse model of SSc. Depletion of IL‐6‐producing Beffs reduced skin and lung fibrosis, while depletion of IL‐10‐producing Bregs caused more severe fibrosis 97 backing up findings from a previous study that Bregs can suppress skin fibrosis in a graft‐versus‐host disease mouse model of SSc 98. A tipping of the balance of Bregs and Beffs in favour of fewer Bregs is therefore a potential important event in the initiation of fibrosis in SSc. However, the cause of this shift in B cell homeostasis remains unknown, and future studies to identify the trigger/s may provide useful early therapeutic targets. Various response regulators on the surface of B cells exist, with some increasing and others decreasing B cell receptor (BCR) signals. Of these, the positive B cell regulator, CD19, is the most established in SSc disease research. CD19 is an important regulatory molecule expressed by B cells. CD19+ B cells increase in SSc, with expression of CD19 on the surface of B cells significantly higher in SSc patients compared to healthy controls 99. CD19‐deficient mice have decreased sera levels of several autoantibodies 100 suggesting that B cells could be responsible for the autoantibodies detected in SSc patients. In agreement, a transgenic mouse model, with an elevation in CD19 expression similar to SSc human patients, shows an increase in the levels of autoantibodies 101, 102. It is also worth noting that CD19 over‐expression has not been observed in the Tsk mouse model, suggesting that a role for CD19 in B cell activation in SSc is not straightforward or that the animal models can only replicate the human disease phenotypes to a degree. However, the apparent lack of CD19 over‐expression may be explained partly through constitutive activation of CD19 and the downstream signalling pathway via up‐regulated CD19 tyrosine phosphorylation, which could negate the need for over‐expression 102. Autoantibody production is prevalent, and can be used diagnostically in SSc. Furthermore, autoantibodies against platelet‐derived growth factor receptor (PDGFR) were found in SSc patients, and caused collagen I‐increased expression and transition of normal human primary fibroblasts to a myofibroblast phenotype through activation of an intracellular loop that involves Ha‐Ras, ERK1/2 and reactive oxygen species (ROS) 103. However, it is still possible that B cell activation is the cause of both autoantibody production and fibrosis, as the two may be linked. Interestingly, B cell activation via CD19 specifically may also be a cause of other immune cell type infiltration in SSc. CD19‐deficient mice showed less infiltration of mast cells, macrophages, T cells and B cells after bleomycin treatment compared to wild‐type cells exposed to bleomycin 104. In the same study, it was discovered that B cells, via hyaluronan‐induced TLR‐4 activation, produced various cytokines which were dramatically reduced in CD19‐deficient cells. Therefore, B cells have the potential to contribute to fibrosis directly as well as indirectly through recruitment and activation of other immune cells to promote a cycle of cytokine and fibrogenic compound release. Mast cells Mast cell infiltration was detected from lip biopsy in very early stages of SSc and preceded the onset of skin changes 105 and more recently cutaneous mastocytosis was found to precede the onset of SSc in a 36‐year‐old woman 106 suggesting that mast cells may play an early role in the onset of SSc. Mast cell infiltration has also been detected late in the development of SSc 107 and in a subset of SSc patients with localized scleroderma‐like lesions 108. An increase in mast cell numbers has also been detected in SSc skin 109. Mast cells are able to produce TGF‐β and are found localized to fibroblasts in the skin of SSc patients 110. Interestingly, mast cells are not only a source of TGF‐β; they can also directly transfer TGF‐β to fibroblasts mediated by transgranulation via cell–cell contacts 111, which has also been observed in the dermis of SSc patients 112. The triggers of mast infiltration in SSc or other fibrotic diseases are not known, but a very recent study has identified Snail‐dependent production of plasminogen activator inhibitor‐1 (PAI1) in keratinocytes as a chemoattractant of mast cells, a mediator of mast cell–fibroblast adhesion and a promoter of fibrogenesis 113. Interestingly, PAI1 up‐regulated tenascin‐C (TENC) expression, which is the main activator of TLR‐4 to promote fibrosis and found to be elevated in SSc 114. Therefore, the role of PAI1 in SSc could involve mast cell recruitment and activation of fibroblasts to promote an inflammatory environment. Dendritic cells Two major types of dendritic cell are known: conventional (cDC) and plasmacytoid (pDC). pDC are a specialized cell type that when activated produce large amounts of interferon (IFN). A very recent study observed that pDCs infiltrate the skin of SSc patients and are chronically activated, producing IFN‐α and chemokine (C‐X‐C motif) ligand 4 (CXCL4). Fibrosis was reverted in a mouse model of SSc after pDCs depletion 115. In the same study, TLR‐8 signalling was found to be important for CXCL4 production and recruitment of pDCs to fibrotic skin. TLR involvement has also been found to be important for mediating the increased cytokine production in DCs in SSc, which has been comprehensively reviewed 116. pDCs isolated from SSc patients show up‐regulation of CXCL4 115, 117, which can also be detected at elevated levels and correlates with disease severity in SSc patient plasma 118 and has been suggested as a biomarker in SSc. In another recent study, pDC depletion in a bleomycin mouse model improved the clinical score, lung histopathology score, skin thickness and collagen content. The expression of genes involved in chemotaxis, dendritic cell differentiation, inflammation and fibrosis in the lungs of pDC‐depleted mice was also significantly reduced. Alongside this, B and T cells were also found to be reduced in the lungs, suggesting an important role for ongoing immune abnormalities induced by DCs 119. In another bleomycin‐induced pulmonary fibrosis mouse model, DC accumulation in the lung was reduced through blocking of TGF‐β with the use of inhibitor SB431542 120. TGF‐β was also found to be important for the recruitment of pDCs (and T cells) to the skin in an SSc mouse model 30 suggesting an important role for TGF‐β in DC location and activation. An important role for microRNAs in the pathogenesis SSc is emerging 121. Recently, microRNA expression profiling has identified miRNA‐618 (miR‐618) as being up‐regulated in pDCs from SSc patients, while over‐expression in healthy pDCs resulted in an SSc‐like pDC 122. Thus, microRNAs are disturbed, leading to altered functionality of immune cells. Alterations of microRNAs by restoration using microRNA therapeutics may be one way to reset dendritic cells to a ‘tolerogenic’ phenotype. Anti‐nuclear antibodies, which are a subset of autoantibodies, are found to be elevated in SSc patients 123. DNA topoisomerase I (TopoI) is an intracellular target of anti‐nuclear antibody anti‐TopoI. The presence of anti‐TopoI antibodies has been clinically associated with a more severe form of SSc 124. Immunization of mice with TopoI peptide‐loaded DCs induces anti‐TopoI autoantibody response and long‐term fibrosis of skin and lung, which is preceded by inflammation with increased IL‐17A and CXCL4 expression 125. TopoI peptide‐loaded DCs also induce proliferation of SSc and healthy‐derived T cells, but activation of T cells requires a fragmented form of TopoI and activation of full‐length TopoI is IL‐2‐dependent 126. Interestingly, antigen‐presenting cells within PBMCs were able to activate T cells more efficiently than dendritic cells, and even processed TopoI peptides differently, suggesting that there is a complex regulation of T cells by dendritic cells and other antigen‐presenting cells with regard to anti‐nuclear antibodies. Overall, experimental manipulation of DCS can mimic some major events in the pathogenesis of SSc, and therefore raises the intriguing prospect that DCs may be capable of initiating SSc. A broken loop that may perpetuate inflammation with cytokine‐mediated up‐regulation of co‐stimulatory molecules may also exist in SSc. Other immune cells Dysregulation and activation of immune cells is clearly a major hallmark of SSc involving many cell types. This complex network of signalling between different immune cells is only now beginning to become unravelled, with new evidence emerging regularly to provide new players in SSc immunopathogenesis. For example, only a few studies have highlighted the potential for platelets 127-129, neutrophils 130, 131, natural killer cells 132, 133 and innate lymphoid cells 134, 135 to be dysregulated and contribute to the pathogenesis of SSc. Type 2 innate lymphoid cells are found in higher numbers in SSc and correlate strongly with the Rodnan skin score and also the presence of lung fibrosis. Although not considered to be innate immune cells, fibroblasts play a crucial role in the fibrotic phenotype of SSc, but they also express TLRs and are therefore capable of responding to DAMPs. Expression of TLR‐2 was found to be increased in fibroblasts from SSc skin 136. Recently, fibroblasts exposed to TGF‐β and IL‐17A responded with a 100‐fold increase in the production of IL‐6 137, a known profibrotic mediator. Conclusion Various cell types of the immune system appear to be involved to some degree in the immunopathogenesis of SSc through promoting other immune cell activation, fibrosis or vascular damage. Very recent studies have highlighted the importance of this plethora of immune cell types in SSc (Table 1). However, research to date provides evidence for macrophages, T cells and B cells having the most important role in SSc. Whether this accurately represents their importance in SSc or is a result of research interests combined with the practicality of investigating specific cell types remains to be seen. Nevertheless, immune cell activation is a recurring observation in SSc research and is leading to the development and testing of therapeutic interventions. Although the over‐production of cytokines is a well‐accepted symptom of SSc, TGF‐β, IL‐6 and IL‐4 specifically seem to be integral to many of the immune abnormalities recorded. Thoroughly understanding how these and other cytokines fully regulate, and are regulated by, all the different immune cells and their subcategories in SSc will be a difficult task, but every study into immune cell activation, regardless of cell type, brings us closer to achieving it and as a result developing therapeutic interventions. Current trials such as the Fassinate trial in SSc blocking IL‐6 seem promising, with the Phase II trial positive, although the primary end‐point was not reached. Phosphodiesterase type 4 (PDE4) inhibitors appear to regulate macrophages leading to reduced fibroblast activation. There is an ongoing trial concerning morphea. Immune cell Recent findings Reference T cell Angiogenic T cells were elevated in SSc patients displaying digital ulcers, which is a severe peripheral vascular complication 59 Macrophage The soluble form of the M2 macrophage marker CD163 is elevated in the serum of patients with SSc, highlighting it as a potential biomarker 66 B cell Used a mouse model of SSc to demonstrate the importance of B cell homeostasis. Depletion of IL‐6‐producing B effector cells reduced fibrosis while depleting of IL‐10‐producing B regulatory cells had the opposite effect 97 Mast cell A subset of SSc patients with localized scleroderma‐like lesions were found to have an inflammatory phenotype leading to the activation of mast cells in the dermis of mechanically stressed skin 108 Dendritic cell After depletion of pDCs fibrosis was reverted in mice with established SSc‐like disease. pDC depletion prior to induction of disease also prevented fibrosis 115 Platelets Activated platelets induced the production of the profibrotic mediator thymic stromal lymphopoietin (TSLP) in human dermal endothelial cells 128 Neutrophils Neutrophil activation was induced by SSc microparticles. Microparticles were derived from platelets and expressed the damage‐associated molecular pattern HMGB1. An inhibitor of HMGB1 attenuated neutrophil activation 131 Natural killer A peculiar natural killer cell phenotype in SSc patients was identified characterized by decreased chemokine and activation receptors expression. These SSc‐derived natural killer cells were potent inducers of endothelial microparticle release suggesting that there may be a role for natural killer cells in the activation of endothelial cells in SSc 133 Innate lymphoid Type 2 innate lymphoid (ILC2) cells are elevated in patients with SSc. ILC2 counts correlated with skin fibrosis. This study highlights that there may be a profibrotic role for ILC2 cells in SSc 134 Fibroblast Although not an immune cell type, fibroblasts exposed to TGF‐β and IL‐17a responded with a 100‐fold increase in the production of IL‐6 137 DCs = dendritic cells; IL = interleukin; TGF = transforming growth factor; HMGB1 = high mobility group box 1. Disclosures None. References

|
Rescooped by
Gilbert C FAURE
from Rheumatology-Rhumatologie
March 4, 2018 4:50 AM
|
The year was 1991. It was my first Tuesday as a rheumatology fellow at the University of Pittsburgh’s Presbyterian Hospital. Navigating a maze of buildings and hallways, I delivered myself to the entrance to the scleroderma clinic. Running late and not knowing whether there was a separate entrance for staff, I clicked open the door.... [Read More]

|
Scooped by
Gilbert C FAURE
August 10, 2017 4:57 AM
|
To mark World Scleroderma Day on 29 June, ‘More Than Systemic Sclerosis: The Inside Story’ launches with a collection of photographic portraits and short films from patients living with systemic sclerosis, also known as scleroderma

|
Scooped by
Anne-Sophie Lagneaux
January 6, 2017 4:49 AM
|
The Scleroderma Foundation is a national nonprofit health organization dedicated to a three-fold mission of Support, Education, and Research to help fight this challenging autoimmune disease.

|
Scooped by
Gilbert C FAURE
May 16, 2016 1:23 PM
|
Objective: Scleroderma patients with autoantibodies to centromere proteins (CENPs) and/or interferon-inducible protein 16 (IFI16) are at increased risk of severe vascular complications.

|
Scooped by
Gilbert C FAURE
December 20, 2015 7:17 AM
|
Objective
To determine the relationships between systemic sclerosis (SSc)–related autoantibodies, as well as their clinical associations, in a well-characterized Australian patient cohort.

|
Scooped by
Gilbert C FAURE
June 25, 2015 8:52 AM
|
Read about how antiphospholipid antibody levels are high in SSc patients.

|
Scooped by
Gilbert C FAURE
March 12, 2015 3:44 PM
|
Examination of autoantibody status & clinical feats that associate w/ cancer risk & cancer-assoc #scleroderma: http://t.co/MYEqbWhd9F

|
Scooped by
Gilbert C FAURE
August 16, 2014 1:42 AM
|
Scleroderma Research Foundation to Host Live Webinar on Diagnosis and ...
Virtual Press Office (press release)
Dr.

|
Scooped by
Gilbert C FAURE
February 18, 2014 1:35 PM
|
Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Pia Moinzadeh, Carmen Fonseca, Martin Hellmich, Ami A Shah, Cecilia Chighizola, Christopher P Denton and Voon H Ong. Author Affiliations.
|

 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...