Your new post is loading...

|
Scooped by
Beeyond
December 29, 2025 7:58 AM
|
The Sapien M3 transcatheter mitral valve replacement (TMVR) system is indicated for the treatment of symptomatic moderate-to-severe or severe MR in patients who are deemed unsuitable for surgery or transcatheter edge-to-edge repair (TEER) therapy by a multidisciplinary heart team.

|
Scooped by
Beeyond
December 29, 2025 7:58 AM
|
Heart valve replacement is a common cardiac procedure, but many patients and families ask one key question: what is the average age for heart valve replacement?
In most cases, heart valve replacement is performed between 60 and 75 years of age, although the exact age varies depending on the valve involved, the underlying heart condition, and the patient’s overall health.

|
Scooped by
Beeyond
December 6, 2025 7:40 AM
|
Artificial intelligence combines ultrasound and X-ray images to give physicians a real-time 3D view of the repair device during transcatheter heart procedures.

|
Scooped by
Beeyond
December 6, 2025 7:39 AM
|
The device, which can be deployed for transcatheter edge-to-edge repair (TEER) in the mitral or tricuspid valves, has similar design features to existing mitral TEER devices including MitraClip (Abbott) and Pascal (Edwards Lifesciences), and includes a mechanical closure system and central spacer. The device has received approval from China’s National Medical Products Administration (NMPA) and CE mark to date.

|
Scooped by
Beeyond
December 6, 2025 7:38 AM
|
The study, which began enrolling in 2015, charts early use of the Intrepid device featuring a transapical delivery system, though later generations of the device have switched to using a transfemoral approach. Procedures were conducted at 21 sites spanning Europe, Australia and the USA and involved 95 patients with symptomatic severe MR who were at high risk for surgery.

|
Scooped by
Beeyond
December 6, 2025 7:38 AM
|
Left ventricular outflow tract (LVOT) obstruction is a common limitation of existing TMVR technologies leading to high rates of screen failure for patients screened for TMVR trials, Ninios highlighted in his presentation, ranking this alongside mitral annulus size and the presence of mitral annular calcification (MAC) as the exclusions that comprise the “Achilles’ heel” of existing TMVR technologies. AltaValve System’s atrial fixation TMVR device is designed to minimise the risk of LVOT obstruction and treat a broad population of mitral regurgitation (MR) patients as well as varied mitral annulus sizes.

|
Scooped by
Beeyond
November 30, 2025 4:14 PM
|
Over the past five years, we observed a 7.24% mortality rate at our tertiary care facility following prosthetic heart valve implantation across all age groups. The data suggest that mortality may be more common among females and older individuals; however, these differences did not reach statistical significance.

|
Scooped by
Beeyond
November 24, 2025 5:53 AM
|
Minimally invasive cardiac procedures such as transcatheter aortic valve replacement (TAVR), catheter-based mitral valve repair, and percutaneous atrial septal defect closure fail at times to attract the same attention given to traditional open-heart surgeries, particularly high-profile procedures such as a coronary artery bypass graft. Studies like this one from the March 2021 issue of the Journal of Thoracic Disease discuss the debate over outcomes, quality, and safety that has followed these procedures.

|
Scooped by
Beeyond
November 17, 2025 9:07 AM
|
Results of a pivotal clinical trial evaluating the safety and efficacy of a fully percutaneous transseptal mitral valve replacement (TMVR) procedure—Sapien M3 (Edwards Lifesciences)—in patients with symptomatic, moderate-to-severe mitral regurgitation (MR) who are not eligible for surgery or mitral-transcatheter edge-to-edge repair (M-TEER) procedures, demonstrated effective MR reduction with low rates of complications and mortality.

|
Scooped by
Beeyond
November 3, 2025 4:22 AM
|
Results from a pivotal clinical trial to evaluate the safety and efficacy of a fully percutaneous transseptal mitral valve replacement (TMVR) procedure in patients with symptomatic, moderate-to-severe mitral regurgitation (MR) who are not candidates for conventional surgery or transcatheter edge-to-edge repair (TEER) procedures, demonstrated effective MR reduction with low rates of complications and mortality.

|
Scooped by
Beeyond
November 3, 2025 4:21 AM
|
In two forms of severe mitral valve disease with high rates of mortality and limited treatment options, multicenter trials with novel transcatheter devices produced what experts described as unprecedented symptom control and rates of survival. The two trials — one directed at severe mitral annular calcifications (MACs) and the other at inoperable severe mitral regurgitation — were conducted without a comparator arm because no meaningful benefit could be expected from alternatives, according to the principal investigators of each trial.

|
Scooped by
Beeyond
November 3, 2025 4:19 AM
|
A moment ten years ago struck me hard and has stayed with me ever since. A nineteen-year-old girl had been diagnosed with rheumatic mitral valve disease. Her family was poor, and a friend brought me her scans. I knew I could save her valve and protect her future. No lifelong anticoagulants. No cold ticking in her chest, like a metronome counting down her days. No fear that a stumble on the stairs might turn into a medical emergency. I told her: make the journey at your own expense, and I will perform the surgery for free. But before I could schedule it, another hospital stepped in. They promised to cover all her hospitalization costs, and then replaced her valve with a mechanical one. She survived. But now, she will live the rest of her life with a foreign device inside her, bearing all the risks and sacrifices that come with it.

|
Scooped by
Beeyond
November 1, 2025 12:37 PM
|
Protembis was founded in 2013 and is headquartered in Aachen, Germany. The company develops innovative technologies to reduce the risk of new brain injury during cardiology procedures particularly in connection with transcatheter aortic valve replacement (TAVR).

|
Scooped by
Beeyond
September 14, 2025 12:21 AM
|
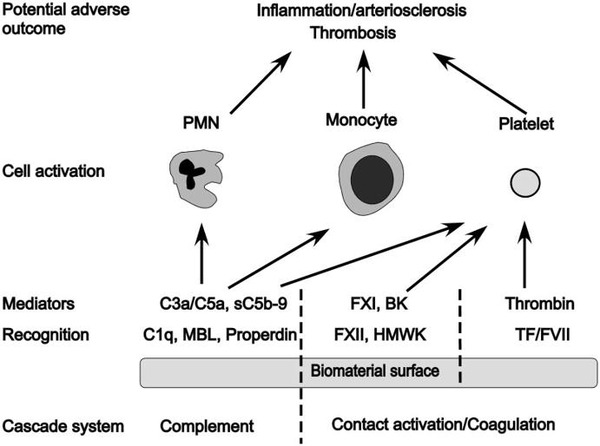
Overall, considerably more success has been achieved in reducing the thrombogenicity of bio-artificial surfaces than in controlling complement activation: For example, surfaces coated with different forms of heparin or PEG are associated with low or negligible activation of coagulation and subsequent platelet loss. Thus, there are numerous surfaces that have low thrombogenicity available that still bear substantial complement-activating capacity. Some of these materials will no doubt be a

|
Scooped by
Beeyond
September 13, 2025 11:52 PM
|
A new-look polymer heart valve is associated with encouraging one-year outcomes in patients undergoing surgical mitral valve replacement (SMVR), according to new data presented at New York Valves 2025 and published in the Journal of the American College of Cardiology.[1]
The Tria mitral valve from Utah-based Foldax is built using LifePolymer, a proprietary material that does not include any animal tissue. Both the frame of the valve and its leaflets are robotically generated to match the patient’s native mitral valve.

|
Scooped by
Beeyond
September 13, 2025 11:51 PM
|
Prosthetic heart valves are classified as Class III medical devices by the FDA; therefore, they require the highest level of regulatory examination before approval for the market by the competent authorities (e.g., the FDA in the US, notified bodies in the EU). For this purpose, the biggest challenge for PHVs is the successful demonstration of long-term durability in vivo: 10–15 years (or more) can be a reasonable timeframe for approval. Moreover, any new prosthetic device has to exhibit better performance compared to the existing ones and, in the specific case of PHVs, the mechanism of failure has to be precisely addressed. All these issues are particularly challenging!

|
Scooped by
Beeyond
September 13, 2025 11:48 PM
|
By the year 2050, it is estimated that over 1 million patients will require a heart valve replacement. The available prosthetic valve options that exist today have several limitations including lifelong anticoagulation for mechanical prostheses and structural valve degeneration associated with bioprosthetic valves. Moreover, the costs and materials required to source and process biological tissue limit the widespread utilization of bioprosthetic valves in low-resource settings. Early trials of polymeric heart valves were unsuccessful largely due to a limited ability to modify polymeric compounds to meet the needs of an ideal heart valve substitute. This review describes several advanced polymer technologies that have been incorporated into more recent PHV models.

|
Scooped by
Beeyond
September 13, 2025 11:45 PM
|
Currently, the problem of structural valve degeneration can be solved only by using either mechanical or biological prostheses. Despite the wide variety of materials and manufacturing technologies discussed in the review, the advancements in this field are limited by the strict requirements for mechanical properties and biostability, the complex and anisotropic behavior of the valve model, and the lack of self-healing synthetic materials. To date, LifePolymer (Foldax) is the only biopolymer material that has successfully passed preclinical tests and has been implanted in humans during clinical trials. However, in light of recent advances in high-molecular compounds and materials science, especially in the development of various copolymers, nanocomposites, and other hybrid structures combining the advantages of compounds, it has become possible to develop patient-specific anisotropic heart valves. New additive manufacturing technologies, such as 3D printing, electrospinning, and microfabrication technologies, have brought the global biomedical community closer to the development of an optimal heart valve prosthesis.

|
Scooped by
Beeyond
September 13, 2025 11:43 PM
|
Traditional valves made from animal tissue are prone to calcification and degradation, limiting their durability and often leading to repeat surgeries, especially for younger patients. Mechanical valves, while durable, are prone to thrombosis and require lifelong anticoagulation therapy that can impact a patient’s long-term quality of life. Foldax’s vision for its novel polymer heart valves is to address the limitations of tissue and mechanical valves by making them durable, with the goal of avoiding the requirement for lifelong anticoagulation.

|
Scooped by
Beeyond
September 11, 2025 11:05 PM
|
Although transcatheter aortic valve replacement thrombosis is a multifactorial process involving foreign materials, patient-specific blood chemistry, and complex flow patterns, our study indicates that deployed THV geometry may have implications on the occurrence of thrombosis. In addition, a supraannular neosinus may reduce thrombosis risk because of reduced flow stasis. Although additional prospective studies are needed to further develop strategies for minimizing thrombus burden, these results may help identify patients at higher thrombosis risk and aid in the development of next-generation devices with reduced thrombosis risk.

|
Scooped by
Beeyond
September 11, 2025 11:01 PM
|
There is a time-dependent degeneration of THVs consisting of thrombus formation, endothelial hyperplasia, fibrosis, tissue remodeling, proteinase expression, and calcification. Future investigation is needed to further understand these mechanisms contributing to leaflet thickening and SVD.

|
Scooped by
Beeyond
August 7, 2025 5:16 PM
|
Degenerative mitral valve disease has doubled in prevalence globally over the last 30 years, and though improvements in surgical techniques and advances in transcatheter technologies have led to improvements in rates of mortality, these have arisen in higher income countries—leading to concern over a global disparity in care for patients with mitral valve disease.

|
Scooped by
Beeyond
August 7, 2025 5:13 PM
|
Patients with heart failure and atrial fibrillation (AF) at the time of mitral transcatheter edge-to-edge repair (M-TEER) for severe mitral regurgitation are more than twice as likely to die or be rehospitalized for heart failure, compared to patients without AF. These are the findings from a Mount Sinai Fuster Heart Hospital study that links the presence of AF at the time of the procedure to worse outcomes following the procedure.

|
Scooped by
Beeyond
June 3, 2025 4:25 AM
|
The Tendyne system replaces mitral valves that are not functioning properly due to a buildup of calcium in the base of the valves, known as severe mitral annular calcification.

|
Scooped by
Beeyond
May 5, 2025 2:18 AM
|
New data from a large, international registry showed balloon-assisted anterior mitral leaflet modification (BATMAN) was safe, effective, and resulted in shorter procedure times among patients undergoing transcatheter mitral valve replacement (TMVR). The data were presented today as late-breaking clinical research at the Society for Cardiovascular Angiography & Interventions (SCAI) 2025 Scientific Sessions.
|

Curated by Beeyond
BEEYOND is a consulting company in the field of disruptive innovation, accompanying established companies on out-of-the-core growth strategy, from creation of new concepts to product launch. Reach us at: contact@beeyond.fr.
|

 Your new post is loading...
Your new post is loading...