When used to edit genomes, Cas9 nucleases produce targeted double-strand breaks in DNA. Subsequent DNA-repair pathways can induce large genomic deletions (larger than 100 bp), which constrains the applicability of genome editing. Here we show that Cas9-mediated double-strand breaks induce large deletions at varying frequencies in cancer cell lines, human embryonic stem cells and human primary T cells, and that most deletions are produced by two repair pathways: end resection and DNA-polymerase theta-mediated end joining. These findings required the optimization of long-range amplicon sequencing, the development of a k-mer alignment algorithm for the simultaneous analysis of large DNA deletions and small DNA alterations, and the use of CRISPR-interference screening. Despite leveraging mutated Cas9 nickases that produce single-strand breaks, base editors and prime editors also generated large deletions, yet at approximately 20-fold lower frequency than Cas9. We provide strategies for the mitigation of such deletions. DNA repair after Cas9-mediated double-strand breaks induces large DNA deletions at frequencies 20-fold higher than elicited by base editors and prime editors leveraging Cas9 nickases producing single-strand breaks.

|
Scooped by
BigField GEG Tech
onto Genetic Engineering Publications - GEG Tech top picks December 3, 2024 6:33 AM
|





 Your new post is loading...
Your new post is loading...














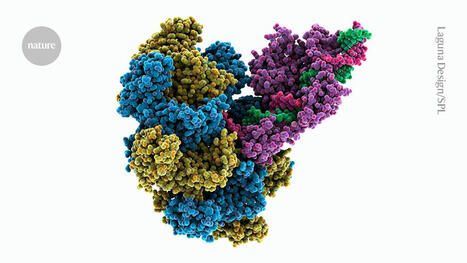






In a paper recently published in Nature Biomedical Engineering, Korean researchers report that large DNA deletions (>100 bp) occur during DNA repair at a much lower frequency for core and major editors than for Cas9 nucleases. The team performed in vitro gene editing on a panel of target sites in various cancer cell lines as well as in human embryonic stem cells and primary human T lymphocytes from two donors. DNA deletions were detected using a combination of CRISPR interference screening and an optimised long-range amplicon sequencing workflow that required the development of a k-mer alignment algorithm to simultaneously analyse large DNA deletions and small indels with high accuracy. The team found that Cas9-mediated double-strand breaks induced large deletions at variable frequencies ranging from 0.2% to 17.5% in all six cell lines, excluding primary T lymphocytes, in which the frequency of large deletions was much higher (around 15% on average for both donors).