 Your new post is loading...
 Your new post is loading...

|
Scooped by
BigField GEG Tech
September 10, 2024 5:06 AM
|
The effectiveness of CAR T cell therapy against a variety of cancers, including solid tumors, could be boosted greatly by using CRISPR-Cas9 technology to knock out the gene for CD5, a protein found on the surface of T cells, according to a preclinical study from investigators at the University of Pennsylvania's Perelman School of Medicine and Abramson Cancer Center.

|
Scooped by
BigField GEG Tech
September 6, 2024 3:50 AM
|
Early findings from a small clinical trial provide evidence that a new cellular immunotherapy approach may be effective in treating metastatic solid tumors. In the trial, researchers from the National Institutes of Health (NIH) genetically engineered normal white blood cells, known as lymphocytes, from each patient to produce receptors that recognize and attack their specific cancer cells.

|
Scooped by
BigField GEG Tech
July 11, 2024 9:12 AM
|
Microbiome research is now demonstrating a growing number of bacterial strains and genes that affect our health1. Although CRISPR-derived tools have shown great success in editing disease-driving genes in human cells2, we currently lack the tools to achieve comparable success for bacterial targets in situ. Here we engineer a phage-derived particle to deliver a base editor and modify Escherichia coli colonizing the mouse gut. Editing of a β-lactamase gene in a model E. coli strain resulted in a median editing efficiency of 93% of the target bacterial population with a single dose. Edited bacteria were stably maintained in the mouse gut for at least 42 days following treatment. This was achieved using a non-replicative DNA vector, preventing maintenance and dissemination of the payload. We then leveraged this approach to edit several genes of therapeutic relevance in E. coli and Klebsiella pneumoniae strains in vitro and demonstrate in situ editing of a gene involved in the production of curli in a pathogenic E. coli strain. Our work demonstrates the feasibility of modifying bacteria directly in the gut, offering a new avenue to investigate the function of bacterial genes and opening the door to the design of new microbiome-targeted therapies. Edited bacteria were stably maintained in mouse gut for at least 42 days following the delivery of a base editor using an engineered phage-derived particle to modify Escherichia coli colonizing the gut.

|
Scooped by
BigField GEG Tech
June 17, 2024 5:05 AM
|
A team at Montana State University published research this week that shows how RNA, the close chemical cousin to DNA, can be edited using CRISPRs.

|
Scooped by
BigField GEG Tech
April 24, 2024 5:41 AM
|
The bioengineered immune players called CAR T cells last longer and work better if pumped up with a large dose of a protein that makes them resemble stem cells.

|
Scooped by
BigField GEG Tech
April 12, 2024 6:58 AM
|
Since its breakthrough development more than a decade ago, CRISPR has revolutionized DNA editing across a broad range of fields.

|
Scooped by
BigField GEG Tech
April 2, 2024 7:26 AM
|
Magnetic resonance imaging (MRI) and lumbar puncture (LP) may not always be necessary for diagnosing and managing a serious neurological complication associated with CAR T-cell therapy, according to a new Blood Advances study.

|
Scooped by
BigField GEG Tech
March 21, 2024 6:48 AM
|
In vivo CRISPR genome-wide screening pinpoints the transcriptional modulator CITED2 as a pivotal driver in the progression of prostate cancer to bone metastasis.

|
Scooped by
BigField GEG Tech
March 11, 2024 6:11 AM
|
Over the past two decades, the immune system has attracted increasing attention for its role in fighting cancer.

|
Scooped by
BigField GEG Tech
March 6, 2024 10:52 AM
|
Current approaches for inserting autonomous transgenes into the genome, such as CRISPR–Cas9 or virus-based strategies, have limitations including low efficiency and high risk of untargeted genome mutagenesis. Here, we describe precise RNA-mediated insertion of transgenes (PRINT), an approach for site-specifically primed reverse transcription that directs transgene synthesis directly into the genome at a multicopy safe-harbor locus. PRINT uses delivery of two in vitro transcribed RNAs: messenger RNA encoding avian R2 retroelement-protein and template RNA encoding a transgene of length validated up to 4 kb. The R2 protein coordinately recognizes the target site, nicks one strand at a precise location and primes complementary DNA synthesis for stable transgene insertion. With a cultured human primary cell line, over 50% of cells can gain several 2 kb transgenes, of which more than 50% are full-length. PRINT advantages include no extragenomic DNA, limiting risk of deleterious mutagenesis and innate immune responses, and the relatively low cost, rapid production and scalability of RNA-only delivery. Transgenes are inserted into human cells by 2-RNA delivery of a retroelement protein and template.

|
Scooped by
BigField GEG Tech
February 7, 2024 6:07 AM
|
study evaluates senolytic CAR T-cell therapy targeting uPAR-positive cells in aged mice, showing its effectiveness in mitigating age-related metabolic dysfunction and offering a potential long-lasting treatment for aging-associated conditions.

|
Scooped by
BigField GEG Tech
February 5, 2024 6:11 AM
|
A new light-inducible RNA base editing tool, padCas13, combines the specificity of CRISPR-Cas13 with the control of light activation and allows for precise, reversible RNA targeting and degradation in mammalian cells, both in vitro and in vivo.

|
Scooped by
BigField GEG Tech
February 1, 2024 11:56 AM
|
Using CRISPR, an immune system bacteria use to protect themselves from viruses, scientists have harnessed the power to edit genetic information within cells.
|

|
Scooped by
BigField GEG Tech
September 9, 2024 5:17 AM
|
CAR-T cell therapy, which targets a specific protein on the surface of cancer cells, causes tumors to shrink or disappear in about half of patients with large B-cell lymphoma who haven't experienced improvement with chemotherapy treatments.

|
Scooped by
BigField GEG Tech
July 31, 2024 9:08 AM
|
Prion disease is caused by misfolding of the prion protein (PrP) into pathogenic self-propagating conformations, leading to rapid-onset dementia and death. However, elimination of endogenous PrP halts prion disease progression. In this study, we describ

|
Scooped by
BigField GEG Tech
June 27, 2024 5:54 AM
|
Genomic rearrangements, encompassing mutational changes in the genome such as insertions, deletions or inversions, are essential for genetic diversity. These rearrangements are typically orchestrated by enzymes that are involved in fundamental DNA repair processes, such as homologous recombination, or in the transposition of foreign genetic material by viruses and mobile genetic elements1,2. Here we report that IS110 insertion sequences, a family of minimal and autonomous mobile genetic elements, express a structured non-coding RNA that binds specifically to their encoded recombinase. This bridge RNA contains two internal loops encoding nucleotide stretches that base-pair with the target DNA and the donor DNA, which is the IS110 element itself. We demonstrate that the target-binding and donor-binding loops can be independently reprogrammed to direct sequence-specific recombination between two DNA molecules. This modularity enables the insertion of DNA into genomic target sites, as well as programmable DNA excision and inversion. The IS110 bridge recombination system expands the diversity of nucleic-acid-guided systems beyond CRISPR and RNA interference, offering a unified mechanism for the three fundamental DNA rearrangements—insertion, excision and inversion—that are required for genome design. A bispecific non-coding RNA expressed by the IS110 family of mobile genetic elements forms the basis of a programmable genome-editing system that enables the insertion, excision or inversion of specific target DNA sequences.

|
Scooped by
BigField GEG Tech
June 17, 2024 4:43 AM
|
A team at Montana State University published research this week that shows how RNA, the close chemical cousin to DNA, can be edited using CRISPRs.

|
Scooped by
BigField GEG Tech
April 15, 2024 9:55 AM
|
Mayo Clinic scientists have developed an immunotherapy strategy that potentially lays the groundwork for treating a spectrum of autoimmune diseases.

|
Scooped by
BigField GEG Tech
April 11, 2024 9:22 AM
|
Treatment with a next-generation CAR T-cell agent displayed early efficacy in a small group of patients with glioblastoma.

|
Scooped by
BigField GEG Tech
March 29, 2024 7:05 AM
|
Targeting two brain tumor-associated proteins-;rather than one-;with CAR T cell therapy shows promise as a strategy for reducing solid tumor growth in patients with recurrent glioblastoma (GBM), an aggressive form of brain cancer, according to early results from the first six patients treated in an ongoing Phase I clinical trial led by researchers from the Perelman School of Medicine at the University of Pennsylvania and Penn Medicine's Abramson Cancer Center.

|
Scooped by
BigField GEG Tech
March 13, 2024 8:21 AM
|
Fluorescence resonance energy transfer (FRET) reporters are commonly used in the final stages of nucleic acid amplification tests to indicate the presence of nucleic acid targets, where fluorescence is restored by nucleases that cleave the FRET reporters. However, the need for dual labelling and purification during manufacturing contributes to the high cost of FRET reporters. Here we demonstrate a low-cost silver nanocluster reporter that does not rely on FRET as the on/off switching mechanism, but rather on a cluster transformation process that leads to fluorescence color change upon nuclease digestion. Notably, a 90 nm red shift in emission is observed upon reporter cleavage, a result unattainable by a simple donor-quencher FRET reporter. Electrospray ionization–mass spectrometry results suggest that the stoichiometric change of the silver nanoclusters from Ag13 (in the intact DNA host) to Ag10 (in the fragments) is probably responsible for the emission colour change observed after reporter digestion. Our results demonstrate that DNA-templated silver nanocluster probes can be versatile reporters for detecting nuclease activities and provide insights into the interactions between nucleases and metallo-DNA nanomaterials. Here the authors present a non-FRET DNA-templated silver nanocluster probe that exhibits a distinct colour switch from green to red upon nuclease digestion, visible under UV excitation, offering a low-cost, effective alternative to fluorescent reporters for detecting nuclease activities.

|
Scooped by
BigField GEG Tech
March 7, 2024 9:49 AM
|
Siteman Cancer Center, based at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, is one of the first centers nationwide to offer a newly approved cell-based immunotherapy that targets melanoma.

|
Scooped by
BigField GEG Tech
March 4, 2024 6:23 AM
|
Review synthesizes research on NK cells' role in cancer immunity and their potential in therapeutics through bioengineering, immune checkpoint inhibitors, and cell engagers, highlighting ongoing preclinical and clinical trials.

|
Scooped by
BigField GEG Tech
February 6, 2024 5:58 AM
|
This study is led by Prof. Xianqun Fan (Department of Ophthalmology, Shanghai Jiao Tong University School of Medicine Affiliated Ninth People's Hospital).

|
Scooped by
BigField GEG Tech
February 2, 2024 6:13 AM
|
Chimeric antigen receptor T-cell therapy, or CAR T, has dramatically improved the treatment of certain blood cancers. Initially approved for patients who had failed multiple lines of therapy, clinical trials have shown CAR T can be used as an earlier treatment option.
|



 Your new post is loading...
Your new post is loading...




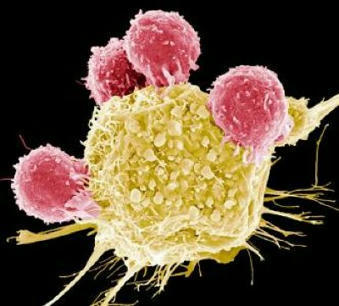











CAR T cells are T cells designed to attack specific targets on cancer cells. They have produced remarkable results in certain patients with blood cancers. However, they have not worked well against other cancers, particularly solid tumor cancers such as pancreatic cancer, prostate cancer, and melanoma. The researchers looked for techniques to increase the effectiveness of CAR T cell therapy. The study, published today in Science Immunology, suggests that using CRISPR-Cas9 technology to knock out the CD5 gene could be a first-rate technique. By shedding light on the hitherto obscure role of the CD5 protein present on the surface of T cells, the researchers discovered that it functions as a robust immune checkpoint, limiting the efficiency of T lymphocytes. By deleting it, they showed that the anti-cancer activity of CAR T cells improved considerably in various preclinical cancer models. A Phase I clinical trial of CD5-knockout CAR T cells will soon begin enrolling patients with CD5-bearing T-cell lymphoma.