A landmark study published in Cell has shown that prime editing, a cutting-edge form of gene editing, can correct mutations causing Alternating Hemiplegia of Childhood (AHC) with a single in-brain injection. The research team fixed the most prevalent ATP1A3 gene mutations in mouse models, reducing symptoms and more than doubling survival, a first-of-its-kind success in treating a neurological disease directly in the brain. CRISPR-based gene editing was delivered through an harmless adeno-associated virus called AAV9. In parallel, patient-derived cells (iPSCs) responded similarly, reinforcing the method’s promise for human translation. Importantly, this success opens the door to targeting other genetic brain disorders previously deemed untreatable. Although results are preliminary, this study provides robust proof‑of‑concept for personalized gene editing in the brain and opens doors toward potential treatments for other intractable genetic neurological disorders.






 Your new post is loading...
Your new post is loading...





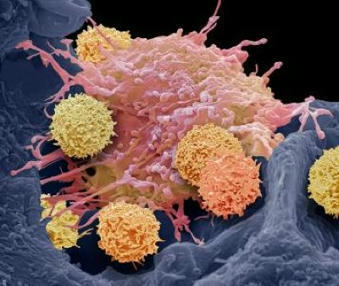


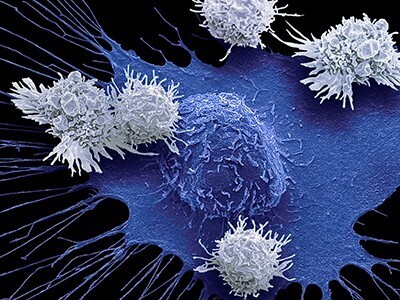













CAR-T cell therapy has proven to be an effective treatment for lymphoma and other blood cancers. However, researchers are struggling to adapt this treatment to solid tumors, including prostate, breast, lung, and ovarian cancers, which account for about 90% of cancer cases. A research team has discovered a promising solution. The researchers genetically modified CAR-T cells to produce a fusion of two proteins: interleukin 12 (IL-12), a cytokine that stimulates immune activity, and a PD-L1 (programmed death ligand 1) inhibitor, an immune checkpoint inhibitor that prevents cancer cells from deactivating the immune response. In mouse models of prostate and ovarian cancer, the modified CAR-T cells triggered a localized attack, reducing the tumor without causing toxicity in other parts of the body. These findings have just been published in the journal Nature Biomedical Engineering.