A landmark study published in Cell has shown that prime editing, a cutting-edge form of gene editing, can correct mutations causing Alternating Hemiplegia of Childhood (AHC) with a single in-brain injection. The research team fixed the most prevalent ATP1A3 gene mutations in mouse models, reducing symptoms and more than doubling survival, a first-of-its-kind success in treating a neurological disease directly in the brain. CRISPR-based gene editing was delivered through an harmless adeno-associated virus called AAV9. In parallel, patient-derived cells (iPSCs) responded similarly, reinforcing the method’s promise for human translation. Importantly, this success opens the door to targeting other genetic brain disorders previously deemed untreatable. Although results are preliminary, this study provides robust proof‑of‑concept for personalized gene editing in the brain and opens doors toward potential treatments for other intractable genetic neurological disorders.





 Your new post is loading...
Your new post is loading...








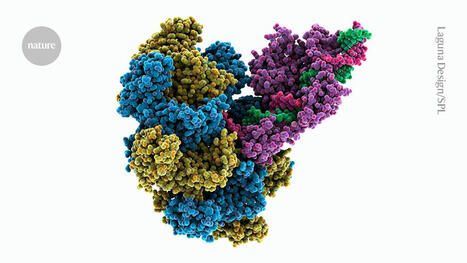













One of the key elements in the use of CRISPR-Cas9 technologies lies in the need for enzymes to locate and bind to a short DNA sequence called the adjacent proto-spacer motif (PAM). The researchers used a machine-learning algorithm, called PAMmla, to predict the PAMs of millions of Cas9 enzymes, thereby identifying a set of novel Cas9 enzymes designed to exhibit the best targeted activity and specificity. The researchers conducted proof-of-concept experiments on human cells and a mouse model of retinitis pigmentosa, and found that the tailored enzymes exhibited greater specificity. This work could help reduce off-target effects, improve the safety and efficacy of editing, and enable researchers to predict customized enzymes for new therapeutic targets.