A landmark study published in Cell has shown that prime editing, a cutting-edge form of gene editing, can correct mutations causing Alternating Hemiplegia of Childhood (AHC) with a single in-brain injection. The research team fixed the most prevalent ATP1A3 gene mutations in mouse models, reducing symptoms and more than doubling survival, a first-of-its-kind success in treating a neurological disease directly in the brain. CRISPR-based gene editing was delivered through an harmless adeno-associated virus called AAV9. In parallel, patient-derived cells (iPSCs) responded similarly, reinforcing the method’s promise for human translation. Importantly, this success opens the door to targeting other genetic brain disorders previously deemed untreatable. Although results are preliminary, this study provides robust proof‑of‑concept for personalized gene editing in the brain and opens doors toward potential treatments for other intractable genetic neurological disorders.






 Your new post is loading...
Your new post is loading...














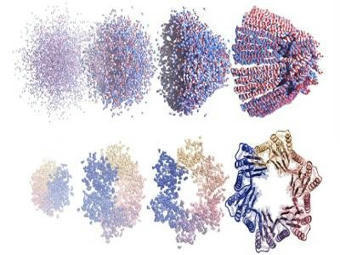





Congenital deafness is an impairment of hearing function due to genetic causes. The GJB2 gene, which codes for connexin 26 (CX26), is responsible for around half of all cases of hereditary deafness. GJB2 mutations often lead to fragmentation of gap junctions and gap junction plaques (GJPs) which are composed of CX26. Japanese researchers have successfully developed a gene therapy to repair the R75W mutation, a dominant-negative mutation in the GJB2 gene responsible for syndromic deafness. The researchers developed an AAV (AAV-Sia6e) capable of delivering genome-editing tools to a wide range of cells in the inner ear that form communicating junctions. The researchers therefore constructed a basic editing tool (SaCas9-NNG-ABE8e) miniaturised to a size allowing it to be transported by the AAV using SaCas9-NNG. Genomic editing via the all-in-one AAV vector showed considerable efficiency and specificity. It enabled targeted conversion of T to C in human cells expressing the GJB2 R75W mutation, repaired this mutation and formed clear GJPs.