A landmark study published in Cell has shown that prime editing, a cutting-edge form of gene editing, can correct mutations causing Alternating Hemiplegia of Childhood (AHC) with a single in-brain injection. The research team fixed the most prevalent ATP1A3 gene mutations in mouse models, reducing symptoms and more than doubling survival, a first-of-its-kind success in treating a neurological disease directly in the brain. CRISPR-based gene editing was delivered through an harmless adeno-associated virus called AAV9. In parallel, patient-derived cells (iPSCs) responded similarly, reinforcing the method’s promise for human translation. Importantly, this success opens the door to targeting other genetic brain disorders previously deemed untreatable. Although results are preliminary, this study provides robust proof‑of‑concept for personalized gene editing in the brain and opens doors toward potential treatments for other intractable genetic neurological disorders.





 Your new post is loading...
Your new post is loading...





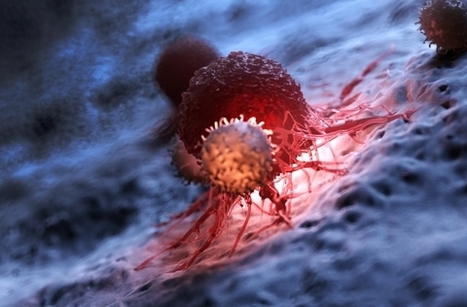
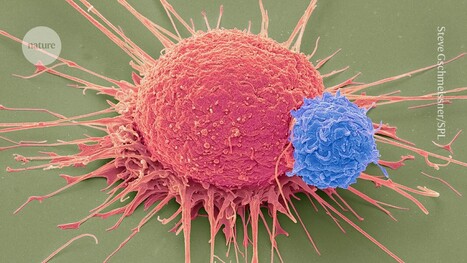






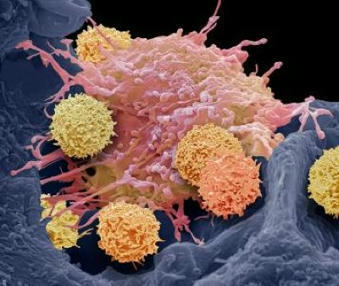


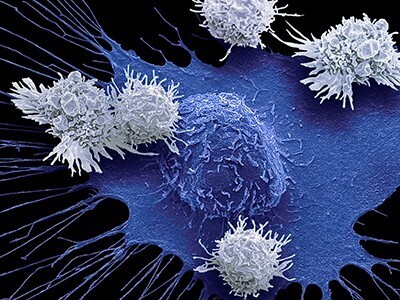







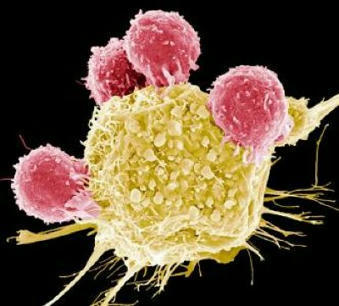
Keeping cells active long enough to eliminate cancer has proved difficult, particularly in solid tumors such as those of the breast and lung. Scientists are therefore looking for better ways to help CAR T cells multiply faster and last longer in the body. To this end, researchers compared samples of CAR T cells used to treat people with leukemia. They analyzed the role of cellular proteins that regulate gene activity and serve as master switches in T cells. They discovered a set of 41 genes that were more active in CAR T cells associated with a good response to treatment than in cells associated with a poor response. All 41 genes appeared to be regulated by a master protein called FOXO1. The researchers then engineered the CAR T cells to produce more FOXO1 than usual. Gene activity in these cells began to resemble that of memory T stem cells, which recognize cancer and respond quickly to it. The researchers then injected the modified cells into mice with different types of cancer. Extra FOXO1 enabled the CAR T cells to better reduce both solid tumors and blood cancers. Moreover, another team also reached the same conclusion by working on gene activity analysis in CAR T cells and also discovered that IL-15 activated genes associated with FOXO1.