The effectiveness of CAR T cell therapy against a variety of cancers, including solid tumors, could be boosted greatly by using CRISPR-Cas9 technology to knock out the gene for CD5, a protein found on the surface of T cells, according to a preclinical study from investigators at the University of Pennsylvania's Perelman School of Medicine and Abramson Cancer Center.

|
Scooped by
BigField GEG Tech
onto Genetic Engineering Publications - GEG Tech top picks September 10, 2024 5:06 AM
|




 Your new post is loading...
Your new post is loading...








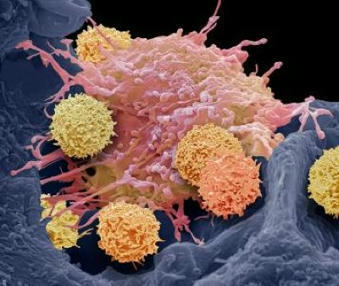


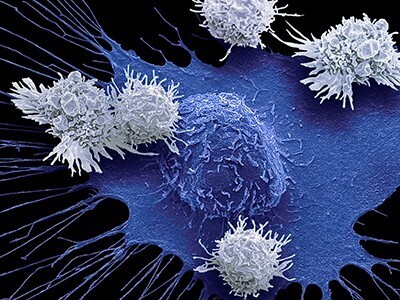






CAR T cells are T cells designed to attack specific targets on cancer cells. They have produced remarkable results in certain patients with blood cancers. However, they have not worked well against other cancers, particularly solid tumor cancers such as pancreatic cancer, prostate cancer, and melanoma. The researchers looked for techniques to increase the effectiveness of CAR T cell therapy. The study, published today in Science Immunology, suggests that using CRISPR-Cas9 technology to knock out the CD5 gene could be a first-rate technique. By shedding light on the hitherto obscure role of the CD5 protein present on the surface of T cells, the researchers discovered that it functions as a robust immune checkpoint, limiting the efficiency of T lymphocytes. By deleting it, they showed that the anti-cancer activity of CAR T cells improved considerably in various preclinical cancer models. A Phase I clinical trial of CD5-knockout CAR T cells will soon begin enrolling patients with CD5-bearing T-cell lymphoma.