 Your new post is loading...
 Your new post is loading...

|
Scooped by
BigField GEG Tech
June 5, 6:56 AM
|
Investigators from Mass General Brigham and Beth Israel Deaconess Medical Center have developed STITCHR, a new gene editing tool that can insert therapeutic genes into specific locations without causing unwanted mutations.

|
Scooped by
BigField GEG Tech
June 2, 11:16 AM
|

|
Scooped by
BigField GEG Tech
May 27, 6:56 AM
|
Imagine a super-charged immune cell that can launch a focused attack on stubborn solid tumors - a smart fighter that destroys cancer cells for days without tiring.

|
Scooped by
BigField GEG Tech
May 20, 5:05 AM
|
‘Directed’ evolution in the laboratory creates an editing tool that outperforms classic CRISPR systems.

|
Scooped by
BigField GEG Tech
May 16, 6:25 AM
|
diagnosis of severe carbamoyl-phosphate synthetase 1 deficiency

|
Scooped by
BigField GEG Tech
April 24, 11:43 AM
|
Congenital hearing loss refers to impaired auditory function that occurs due to genetic causes. GJB2 is the gene responsible for approximately half of all cases of hereditary hearing loss.

|
Scooped by
BigField GEG Tech
April 3, 7:10 AM
|
Chimeric antigen receptor (CAR) T cells and bispecific T cell engagers have become integral components in the treatment of relapsed/refractory multiple myeloma. We report a 63-year-old male who received ciltacabtagene autoleucel CAR T cells and the GPRC5D × CD3 bispecific talquetamab for early relapse of his multiple myeloma. Nine months after CAR T therapy, he developed a symptomatic leukemic peripheral T cell lymphoma with cutaneous and intestinal involvement. Longitudinal single-cell RNA and T cell receptor sequencing of peripheral blood and bone marrow revealed two hyperexpanded CAR-carrying T cell clones. These expanded clones exhibited an exhausted effector-memory T cell transcriptional signature, and the neoplasm itself was sensitive to dexamethasone treatment. The immunophenotypic and transcriptional alterations of these abnormal T cells resembled those of T-large granular lymphocytic leukemia. Spatial transcriptomes of skin lesions confirmed the aberrant CAR-expressing T cells. Whole-genome sequencing revealed three distinct integration sites, within the introns of ZGPAT, KPNA4 and polycomb-associated noncoding RNAs. Before and after CAR T whole-genome analyses implicated clonal outgrowth of a TET2-mutated precursor propelled by additional subclone-specific loss of heterozygosity and other secondary mechanisms. This case highlights the evolution of a CAR-carrying peripheral T cell lymphoma following CAR T cell and bispecific T cell engager therapy, offering critical insights into the clonal evolution from a predisposed hematopoietic precursor to a mature neoplasm. A longitudinal multiomics analysis of a patient with multiple myeloma who developed peripheral T cell lymphoma after treatment with anti-BCMA CAR T cells and a GPRC5D-directed bispecific antibody reveals that two mutated CAR+CD8+ T cell clones were probably drivers of the neoplasm.

|
Scooped by
BigField GEG Tech
March 25, 3:22 AM
|
Abstract. Human trisomy 21, responsible for Down syndrome, is the most prevalent genetic cause of cognitive impairment and remains a key focus for prenatal

|
Scooped by
BigField GEG Tech
March 10, 7:11 AM
|
Cas12a is a next-generation gene editing tool that enables multiplexed gene targeting. Here, we present a mouse model that constitutively expresses enhanced Acidaminococcus sp. Cas12a (enAsCas12a) linked to an mCherry fluorescent reporter. We demonstrate efficient single and multiplexed gene editing in vitro, using primary and transformed cells from enAsCas12a mice. We further demonstrate successful in vivo gene editing, using normal and cancer-prone enAsCas12a stem cells to reconstitute the haematopoietic system of wild-type mice. We also present compact, genome-wide Cas12a knockout libraries, with four crRNAs per gene encoded across one (Scherzo) or two (Menuetto) vectors, and demonstrate the utility of these libraries across methodologies: in vitro enrichment and drop-out screening in lymphoma cells and immortalised fibroblasts, respectively, and in vivo screens to identify lymphoma-driving events. Finally, we demonstrate CRISPR multiplexing via simultaneous gene knockout (via Cas12a) and activation (via dCas9-SAM) using primary T cells and fibroblasts. Our enAsCas12a mouse and accompanying crRNA libraries enhance genome engineering capabilities and complement current CRISPR technologies. Cas12a represents the next generation of gene editing. Here, the authors present the generation and validation of a Cas12a transgenic mouse model. Additionally, the authors create whole-genome Cas12a knockout libraries, and demonstrate their utility across multiple in vitro and in vivo screens.

|
Scooped by
BigField GEG Tech
February 20, 12:00 PM
|
CAR T cell therapy is one of the most promising new cancer treatments to emerge in recent years. It involves removing a patient's own immune T cells and engineering them to recognize specific targets on the surface of the cancer cell.

|
Scooped by
BigField GEG Tech
January 20, 7:03 AM
|
An emerging class of anticancer drugs called EZH2 inhibitors may greatly enhance the potency of some cancer immunotherapies, according to a preclinical study led by Weill Cornell Medicine lymphoma researchers.

|
Scooped by
BigField GEG Tech
January 13, 11:02 AM
|
Researchers have found a way to program immune cells to attack glioblastoma and treat the inflammation of multiple sclerosis in mice.

|
Scooped by
BigField GEG Tech
December 9, 2024 7:03 AM
|
As cancer cells grow, they pump out metabolic byproducts such as lactic acid into the tumor microenvironment. Exhausted T cells -; which have lost their cancer-fighting oomph -; consume this lactic acid, which further saps their energy, according to new research from the University of Pittsburgh and UPMC Hillman Cancer Center.
|

|
Scooped by
BigField GEG Tech
June 4, 6:46 AM
|
Trial in China is one of the first times the immune therapy has worked against solid tumours.

|
Scooped by
BigField GEG Tech
June 2, 7:13 AM
|
A new review outlines genetic engineering strategies, including CRISPR, to enhance the therapeutic potential of innate immune cells in cancer immunotherapy. The authors evaluate preclinical studies and emerging clinical data on how these cells can be retooled to overcome the limitations of conventional CAR T cell therapy.

|
Scooped by
BigField GEG Tech
May 21, 10:22 AM
|

|
Scooped by
BigField GEG Tech
May 19, 11:55 AM
|
Genome editing has advanced at a rapid pace with promising results for treating genetic conditions-but there is always room for improvement.

|
Scooped by
BigField GEG Tech
May 5, 9:01 AM
|
Scientists deploy self-splicing protein subunits to insert strange new additions into target proteins.

|
Scooped by
BigField GEG Tech
April 11, 5:57 AM
|
Neurons engineered to evade the immune system could work as cell-replacement therapy.

|
Scooped by
BigField GEG Tech
April 2, 11:20 AM
|
A groundbreaking study in Nature Medicine reveals that GD2 CAR-T cell therapy can lead to long-term remission in children with neuroblastoma, with one patient remaining cancer-free for over 18 years.

|
Scooped by
BigField GEG Tech
March 19, 7:00 AM
|
The bioplastic was malleable, but is more expensive to produce than are plastics made from fossil fuels.

|
Scooped by
BigField GEG Tech
March 4, 6:42 AM
|
The programmable proteins are compact, modular, and can be directed to modify DNA in human cells.

|
Scooped by
BigField GEG Tech
February 10, 5:50 AM
|
Findings from a multi-institutional, international study led by researchers from the Mayo Clinic Comprehensive Cancer Center have significantly advanced the understanding of genetic alterations in the BRCA2 gene, a key player in hereditary cancer risk.

|
Scooped by
BigField GEG Tech
January 16, 6:51 AM
|
The DNA-PKcs inhibitor AZD7648 enhances CRISPR–Cas9-directed homology-directed repair efficiencies, with potential for clinical utility, but its possible on-target consequences are unknown. We found that genome editing with AZD7648 causes frequent kilobase-scale and megabase-scale deletions, chromosome arm loss and translocations. These large-scale chromosomal alterations evade detection through typical genome editing assays, prompting caution in deploying AZD7648 and reinforcing the need to investigate multiple types of potential editing outcomes. A compound that enhances homology-directed repair in CRISPR editing leads to genome instability.

|
Scooped by
BigField GEG Tech
January 6, 7:09 AM
|
Researchers developed SNIPRs, innovative receptors that sense soluble ligands, enabling precise therapeutic control in CAR T-cells. This breakthrough enhances tumor targeting while reducing off-target effects, paving the way for advanced synthetic biology applications.
|





 Your new post is loading...
Your new post is loading...






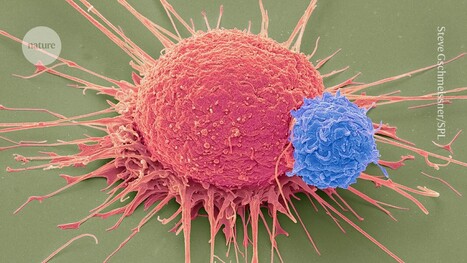









Neuroblastoma, a highly aggressive childhood cancer, presents a high risk of relapse, making long-term disease control a significant challenge. A groundbreaking study published in Nature Medicine has demonstrated that GD2-directed CAR-T cell therapy can induce durable remissions, with one patient remaining cancer-free for over 18 years, the longest reported remission for a solid tumor treated with CAR-T cells. Between 2004 and 2009, 19 children were infused with CAR-T cells targeting GD2, a protein expressed in neuroblastoma cells, as part of a phase I clinical trial. Despite lacking modern co-stimulatory molecules, these first-generation CAR-T cells persisted for over five years in some patients. Among patients with active disease, 3 achieved complete responses, with 2 maintaining long-term remissions of 8+ and 18+ years, respectively. Immune profiling of long-term survivors revealed that CAR-T cells exhibited a mix of effector and memory-like properties, suggesting a key role in extended therapeutic effects. This study indicates the safety and durability of GD2 CAR-T therapy in neuroblastoma and paves the way for improved CAR-T strategies targeting solid tumors.