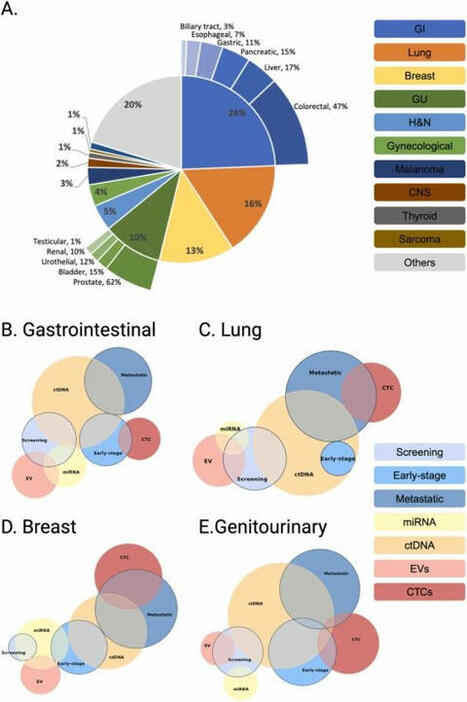
Abstract Background The aim of this study was to determine a correlation between benign and malignant lung solitary pulmonary nodules (SPN), and analyze the association between circulating tumor cell (CTC) levels and different subtypes of lung adenocarcinoma. Methods A total of 200 patients (80 with SPNs and 120 diagnosed with lung cancer) were included in the study. The CTC levels were quantified by identifying the folate receptor on the surface of tumor cells; clinical tumor specific markers were detected by biochemical immunization. The content of peripheral blood CTCs in benign and malignant lung SPN patients was detected and the differences in preoperative CTC levels in different pathological subtypes were analyzed. Based on the collected data, receiver operating characteristic curves were calculated and the rate of lung cancer was predicted. Results The peripheral blood CTC levels in patients with malignant lung SPNs were higher than in patients with benign SPNs. The maximum nodule diameter, carcinoembryonic antigen, and CTC levels were independent risk factors for malignant lung SPNs. The peripheral blood CTC levels in patients with stage III–IV lung adenocarcinoma were higher than in stage I–II patients. The peripheral blood CTC levels in patients with microinvasive and invasive adenocarcinoma were higher than in adenocarcinoma in situ patients. The CTC levels in the peripheral blood of patients with maximum tumor diameter > 2 cm were higher than in patients with tumors < 2 cm. Conclusion The detection of CTCs can be used as a biomarker for screening SPNs and diagnosing early‐stage lung cancer. Using the combination of CTC levels and CEA significantly improves the efficacy of lung adenocarcinoma diagnosis. Introduction Lung cancer is a significant malignancy; United States (US) cancer statistics estimate that 83 550 men and 70 500 women will die from lung cancer in 2018, ranking first of all malignancies.1 Although methods for early screening, diagnosis, and multidisciplinary treatment have been developed, the overall survival rate of lung cancer patients has not greatly improved and patients are usually diagnosed in late stages. Even if surgery is performed at an early stage of the disease, there is still a 25–50% recurrence rate.2 Solitary pulmonary nodules (SPNs) are single, well‐defined lesions of a diameter of ≤ 3 cm that are completely surrounded by gas‐containing lung tissue, without pulmonary atelectasis, hilar enlargement, or pleural effusion.3 At present, SPNs are a focus of research, primarily related to imaging, biomarkers, and histopathology. There are few biomarkers for lung cancer during early stage, thus it is difficult for clinicians to determine optimal treatment.4 With advances in precision medicine and progress in technology, the detection of circulating tumor cells (CTCs) in peripheral blood is a simple and noninvasive “liquid biopsy” method.5 CTCs can be detected earlier than imaging and clinical symptoms. They are collectively referred to tumor cells, metastasize in blood circulation, and may lead to tumor progression and spread throughout the body. In addition, they are often used as a predictor in lung cancer patients for progression‐free and overall survival.6 Real‐time dynamic analysis of CTCs may provide a new metric for the individualized treatment of cancer patients.7 More importantly, this method has become important to evaluate the status of tumors.8 Folate receptor (FR) is highly expressed in many epithelial‐derived malignant tumor cells, and is significantly upregulated in 75.7% of non‐small cell lung cancer (NSCLC) patients.9 There are approximately 500 000 receptors on the surface of each lung cancer cell, but no expression in normal human blood cells. Consequently, FR is an ideal detection point for CTCs in patients with lung cancer. FR‐positive CTCs can be detected by ligand‐targeted enzyme‐linked polymerization4 and have shown diagnostic promise for various cancers.10 For instance, FR expression is higher in both the serum and urine specimens of patients with bladder transitional cell carcinoma compared to a control group.11 FR‐positive CTCs are used as a novel diagnostic biomarker for lung cancer.12 Therefore, we hypothesized that the presence of FR‐positive CTCs were related to the pathological type, stage, and size of lung tumors. In this study, identification of FR in tumor cells was used to quantify CTC levels in benign and malignant SPNs and for the early detection and adjuvant diagnosis of lung cancer. Methods Case selection In this prospective clinical study, 200 patients (80 with SPNs and 120 with lung cancer) diagnosed at the Department of Respiratory Medicine, Thoracic Surgery, and Oncology of the First Affiliated Hospital of Soochow University between September 2016 and June 2017, were enrolled. All patients had a histopathological diagnosis report. Clinical tumor node metastasis (TNM) staging was confirmed based on 7th edition International Association for the Study of Lung Cancer guidelines (2009).13 All patients (aged 18–80 years) included had good compliance, had not received any anti‐tumor therapy, and did not have other types of malignant tumors, or abnormal liver and kidney function. Patients voluntarily participated in the study and signed informed consent. The ethics committee of the First Affiliated Hospital of Soochow University approved the study. Detection of folate receptor (FR)‐positive circulating tumor cells (CTCs) A total of 3 ml antecubital vein blood was extracted from each patient, anticoagulated by ethylene‐diamine‐tetraacetic acid, and stored at 4°C. Blood treatment was completed within 24 hours. CTCs were captured in peripheral blood by immunomagnetic bead negative enrichment and FR‐positive CTCs were quantitatively detected by ligand‐targeted PCR. GenoBiotech Co., Ltd (Shanghai, China) provided an FR‐positive CTC test kit. One FR‐positive cell detected in 3 mL of blood was defined as 1 folate unit (FU).12 Single blind protocols were followed in this study. According to kit instructions, red blood cell lysate (1:4) was added to 3 mL whole blood samples and lysed at 4°C for 15 minutes to remove red blood cells. Then, 150 μL anti‐CD45 magnetic beads and 50 anti‐CD14 magnetic beads were added and incubated for 30 minutes at 4°C to remove leukocytes and macrophages, respectively. The enriched CTCs were added to 10 μL of the probe labeling solution, which contained the tumor‐specific folate ligand‐oligonucleotide conjugate, and incubated at room temperature for 40 minutes. Washing buffer (1 mL) was then added into the samples, which were centrifuged at 4°C and 500 g for 10 minutes three times to remove unbound probes. Finally, 120 μL of elution buffer was added and incubated for 2 minutes at 4°C to elute the bound probe. The bound probe was collected by centrifugation and 24 μL neutralization buffer was added for amplification of fluorescence quantitative PCR. ABI7300 instruments (Life Technologies, Carlsbad, CA, USA) were used to collect PCR signals and data. The reaction conditions were: 95°C, 2 minutes; 40°C, 30 seconds; 72°C, 30 seconds; 8°C, 5 minutes; 40 cycles, 95°C, 10 seconds; 35°C, 30 seconds; and 72°C, 10 seconds.14 Detection of clinical tumor‐specific biomarkers Gastrin releasing peptide (pro‐GRP) was detected by chemiluminescent microparticle immunoassay (CMIA); pro‐GRP in human serum was quantitative determined on the ARCHITECTi system (Abbott Laboratories, Chicago, IL, USA). The normal reference range of pro‐GRP is 0–70 pg/mL. A Roche Cobas 601 automatic chemical analyzer (Roche, Basel, Switzerland) was used to detect the expression of serum tumor biomarkers, including neuron‐specific enolase (NSE), carcinoembryonic antigen (CEA), cytokeratin fragment 19 (CYFRA21‐1), and squamous cell carcinoma (SCC) antigen. The normal reference ranges of these biomarkers were: CEA < 5.0 μg/L, CYFRA21‐1 < 3.3 μg/L, NSE < 16.3 μg/L, SCC < 1.5 μg/L, and pro‐GRP < 70 pg/mL. Statistical analysis SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The continuous variables that corresponded to normal distribution are expressed as mean ± standard deviation, continuous variables that did not follow normal distribution are expressed as medians, and categorical variables are expressed as frequency (%). Comparison of the measurement data between the groups was performed using an independent t‐test. The non‐parametric rank sum test was used for data of non‐normal distribution. The Enter method of logistic regression analysis was used for independent analysis of influencing factors: A receiver operating characteristic (ROC) curve was plotted with sensitivity as the ordinate and specificity as the abscissa. The Youden index was calculated using the ROC curve. The highest cutoff point of the Youden index was determined to be the best critical point for a diagnosis of lung cancer by FR‐positive CTCs. Results CTC levels in patients with benign malignant lung nodules Of the 80 patients with SPNs included in the study, 50 were confirmed to have malignant SPNs (62.5%): 32 had adenocarcinoma, 8 SCC, 5 small cell lung cancer (SCLC), and 1 had a carcinoid. The remaining 30 (32%) patients had benign SPNs: 20 had hamartomas, 4 inflammatory pseudotumors, 3 sclerosing hemangiomas, and 3 had tuberculosis. Patient characteristics by SPN and CTC levels are listed in Tables 1 and 2, respectively. Variables Patients with benign SPN (n = 30) Patients with malignant SPN (n = 30) Age (years) ≤ 60 12 15 > 60 18 35 Gender Male 8 27 Female 22 23 Smoking history Yes 8 30 No 22 20 The largest diameter of nodules (cm) ≤ 0.8 20 14 0.9–3.0 10 36 CEA Normal 22 10 Abnormal 8 40 SCC Normal 16 21 Abnormal 14 29 Pro‐GRP Normal 18 24 Abnormal 12 26 CYFRA21‐1 Normal 14 14 Abnormal 16 36 NSE Normal 13 18 Abnormal 17 32 Number of patients with CTCs Normal 23 15 Abnormal 7 35 CEA, carcinoembryonic antigen; CTC, circulating tumor cell; CYFRA, cytokeratin fragment 19; GRP, gastrin releasing peptide; NSE, neuron‐specific enolase; SCC, squamous cell carcinoma; SPN, solitary pulmonary nodule. Clinical pathological factors CTC level, median (quartile) P Age (year) 0.828 ≤ 60 8.10 (7.43–9.67) > 60 8.35 (7.80–9.57) Gender 0.903 Male 9.05 (8.49–10.52) Female 8.10 (7.21–9.01) Smoking history 0.861 Yes 10.10 (8.74–10.49) No 8.10 (6.804–8.755) Largest diameter of nodules (cm) 0.341 ≤ 0.8 8.30 (7.39–9.38) 0.9–3.0 8.47 (7.88–9.79) CTC, circulating tumor cell. To evaluate the diagnostic efficacy of FR‐positive CTCs for the early diagnosis of lung cancer, the data of malignant SPN patients were analyzed using the Mann–Whitney U test. As shown in Figure 1, the median CTC level in malignant SPN patients was 9.79 (range: 8.98–10.60) units, which was significantly higher than 6.66 (range: 5.81–7.51) in patients with benign SPN disease. The difference was statistically significant (P < 0.001). Multivariate logistic regression analysis was performed to determine what factors were predictive of cancer. Benign and malignant SPNs were used as the dependent variable and each demographic factor was used as an independent variable. The maximum diameter of nodules, CEA, and CTC levels were independent risk factors for malignant SPN disease (P < 0.05) (Table 3). Variables β SE Wald χ2 P OR 95% CI CTCs 1.969 0.760 6.709 0.010 7.162 1.615–31.772 CEA 1.724 0.708 5.929 0.015 5.606 1.400–22.450 SCC 0.704 0.783 0.810 0.368 2.022 0.436–9.380 NSE 0.392 0.680 0.333 0.564 1.480 0.391–5.607 CYFRA21‐1 1.288 0.872 2.182 0.140 3.624 0.657–2.100 Pro‐GRP 0.280 0.808 0.120 0.729 0.755 0.155–3.682 Largest nodule diameter 1.590 0.716 4.931 0.026 4.903 1.205–19.948 CI, confidence interval; CEA, carcinoembryonic antigen; CTC, circulating tumor cell; CYFRA, cytokeratin fragment 19; GRP, gastrin releasing peptide; NSE, neuron‐specific enolase; OR, odds ratio; SCC, squamous cell carcinoma; SE, standard error. Differences in peripheral blood CTC levels in lung cancer patients Of the 120 patients with lung adenocarcinoma included in the study, 40 had adenocarcinoma in situ (AIS), 41 had microinvasive adenocarcinoma (MIA), 23 had invasive adenocarcinoma (IA), and 16 had IA mutations. Based on TNM staging, 41 patients were at stage I, 36 at stage II, 33 at stage III, and 10 patients were at stage IV. Furthermore, 58 patients had highly differentiated tumors, 25 had moderately differentiated tumors, and 17 had poorly differentiated tumors. The other factors are shown in Table 4. Clinical pathological factors Cases (n = 120) Average age (years) 59.4 (25–85) Gender: (male/female) 49/71 Smoking history 61 (50.8%) Largest diameter of nodules (cm) 4.2 cm (0.7–12) T staging T1 63 (52.5%) T2 18 (15.0%) T3 23 (19.2%) T4 10 (8.3%) N staging N0 41(34.2%) N1 37 (30.8%) N2 36 (30.0%) N3 6 (5%) M staging M0 110 (91.7%) M1 10 (8.3%) Histological grade G1 41 (34.2%) G2 46 (38.3%) G3 33 (27.5%) TNM staging I 41 (34.2%) II 36 (30.0%) III 33 (27.5%) IV 10 (8.3%) Pathological subtype Adenocarcinoma in situ 40 (33.3%) Microinvasive adenocarcinoma 41 (34.2%) Invasive adenocarcinoma 23(19.2%) Invasive adenocarcinoma variants 16(13.3%) TNM, tumor node metastasis. As shown in Figure 2 and Table 5, the median CTC level in the lung adenocarcinoma group was 10.65 units (range: 9.93–11.37), and 6.66 (5.81–7.51) in the benign SPN group. The results of the Mann–Whitney U test showed that CTC levels in the lung adenocarcinoma group were higher than in the benign lung SPN group, and the difference was statistically significant (P < 0.001). In patients with lung adenocarcinoma, CTC levels in the peripheral blood of primary tumors with a maximum diameter > 2 cm were higher than those with a maximum diameter of ≤ 2 (P < 0.001). CTC levels in patients at stage III–IV lung adenocarcinoma were significantly higher than patients at stage I–II (P < 0.001). The levels of peripheral blood CTCs in patients with MIA and IA were significantly higher than in patients with AIS (MIA and IA together versus AIS: P < 0.03, IA: P < 0.002). Clinicopathological factors CTC level, median (quartile) P Average age (years) 0.662 ≤ 60 10.42 (8.98–11.87) > 60 10.24 (9.94–11.59) Gender 0.872 Male 10.72 (9.56–11.89) Female 10.60 (9.67–11.54) Smoking history 0.366 Yes 10.97 (9.89–12.05) No 10.31 (9.35–11.28) The largest diameter of nodules (cm) ≤ 2 cm 9.22 (8.06–10.38) 0.001 > 2 m 11.56 (10.69–12.43) Histological grade G1 10.27 (9.33–11.21) 0.593 G2 10.59 (9.31–11.87) G3 11.21 (9.58–12.84) TNM staging I and II staging 9.08 (8.47–9.69) 0.000 III and IV staging 13.49 (12.13–14.84) Pathological subtype Adenocarcinoma in situ 9.18 (8.29–10.06) 0.015 Microinvasive adenocarcinoma 11.07 (9.97–14.45) Invasive adenocarcinoma 12.38 (10.32–14.45) Invasive adenocarcinoma variants 10.77 (7.99–13.56) CTC, circulating tumor cell; TNM, tumor node metastasis. Receiver operating characteristic curve analysis To further analyze the potential value of FR‐positive CTC detection methods to diagnose lung cancer, all patients with lung cancer were included as a case group and patients with benign SPN as a control to create an ROC curve. Using sensitivity as the ordinate and 1‐specificity as the abscissa, ROC analysis demonstrated an area under the curve (AUC) of 0.836 and a 95% confidence interval (CI) 0.770–0.902). The Youden index was utilized to select the best cutoff value. As demonstrated in Figure 3, when sensitivity + specificity‐1 reached a maximum, the CTC value was 8.35, and a CTC level > 8.35 units was positive. Taking 8.35 units as the cutoff value for diagnosis, the diagnostic sensitivity of lung cancer patients was 70.2%, with a specificity of 79.3%. To further analyze the efficacy of FR‐positive CTC detection in patients with different pathological types of lung adenocarcinoma, the sensitivity in patients with AIS, MIA, invasive glands, and IA variants were 60%, 73.2%, 73.9%, and 75%, respectively. Effect of combining logistic regression multivariate analysis of CTCs and clinical biomarkers for the detection of lung adenocarcinoma Benign lung SPN and lung adenocarcinoma were used as dependent variables, while CTC levels and clinical biomarkers were used as independent variables for multivariate logistic regression analysis. As shown in Table 6, CTCs and CEA were independent risk factors for lung adenocarcinoma (P < 0.021 and P < 0.001, respectively). Variables β SE Wald χ2 P OR 95% CI CTCs 1.256 0.543 5.361 0.021 3.512 1.213–10.169 CEA 1.710 0.517 10.95 0.001 5.528 2.008–15.221 SCC 0.064 0.507 0.016 0.900 1.066 0.394–2.881 NSE 0.234 0.439 0.283 0.595 10 263 0.534–2.990 CYFRA21‐1 0.483 0.493 0.959 0.327 1.621 0.616–4.263 Pro‐GRP 0.304 0.491 0.384 0.535 1.356 0.518–3.550 Tumor size 1.005 0.533 3.919 0.058 2.873 1.011–8.167 CI, confidence interval; CEA, carcinoembryonic antigen; CTCs, circulating tumor cells; CYFRA, cytokeratin fragment 19; GRP, gastrin releasing peptide; NSE, neuron‐specific enolase; OR, odds ratio; SCC, squamous cell carcinoma; SE, standard error. Discussion In 1869, an Australian physician, Thomas Ashworth, dissected a patient with advanced stage cancer and accidentally discovered cells in his peripheral blood that resembled the size and shape of the primary tumor, and subsequently proposed the concept of circulating tumor cells (CTC).15 Based on Ashworth's discovery, British pathologist, Stephen Paget proposed the well‐known hypothesis of seed and soil in 1889, successfully explaining the mechanism of tumor recurrence and metastasis.16 Choosing the right tumor‐specific antigen target is key to the success of CTC detection technology. Today, conventional hematology and high‐resolution imaging tests are still inadequate to allow early diagnosis, evaluation of efficacy, assessment of prognosis, and individualized treatment of lung cancer patients. Fortunately, CTC detection, which measures metastasis and recurrence, could make up for the deficiencies of these other detection methods. The results of this study were consistent with our speculation, that peripheral blood CTCs levels in patients with stage III–IV lung adenocarcinoma were higher than in stage I–II patients. The peripheral blood CTC levels in patients with MIA and IA were higher than those in patients with AIS. The CTC levels in the peripheral blood of patients with maximum tumor diameter (MTD) > 2 cm were higher than in patients with MTD < 2 cm. Carcinoembryonic antigen is one of the most widely used tumor biomarkers and the most valuable indicator to diagnose lung adenocarcinoma. CYFRA21‐1, a soluble fragment of cytokeratin 19 is mainly suitable for the early diagnosis of NSCLC, observation of efficacy, and evaluation of prognosis, especially for SCC with relatively high sensitivity and specificity.17 Pro‐GRP is a neuropeptide hormone, one of the molecules associated with neuroendocrine‐derived tissues and tumors. It is usually secreted by SCLC cells, acting as a tumor biomarker of SCLC, and contributes to the differential diagnosis of SCLC and NSCLC.18 Moreover, NSE is the key enzyme for adenosine triphosphate synthesis in glycolysis and is considered the most sensitive and specific biomarker for SCLC.19 The FR has a strong specificity for the tissue of a tumor and is highly expressed in lung cancer cells, particularly on the surface of NSCLC cells. The FR level in each cell surface is up to hundreds of thousands, while almost no FR expression is found in healthy human blood cells.20 Therefore, FR it is an ideal screening target for CTCs. A combination of FR‐positive CTC detection kits, enriched immunomagnetic bead negative screening, and targeted PCR technology could be used for the auxiliary diagnosis of various types and stages of lung cancer. In this study, the sensitivity of FR‐positive CTCs in the peripheral blood of patients with benign and malignant SPNs was evaluated. The average CTC level in malignant lung SPN patients was 9.79 (8.98–10.60) units, which was significantly higher than the average of 6.66 (5.81–7.51) in benign SPN patients. In addition, multivariate regression analysis showed that CTC level, CEA, and maximum nodule diameter were independent risk factors for malignant lung cancer patients, suggesting that detecting CTC levels combined with CEA could improve diagnostic efficacy for malignant lung SPNs. Analysis of these results revealed that the detection methods of FR‐positive CTCs can be used to screen biomarkers to isolate pulmonary nodules and diagnose early lung cancer. In a previous study, CTCs and serum/plasma markers were used as prognostic and predictive biomarkers for early‐stage NSCLC.21 Interestingly, detecting CEA/CK/CD133 messenger RNA in tumor drainage blood is reported to have prognostic value in CRC patients with Dukes’ stage B and C.22 Furthermore, breast cancer could be classified and individualized by detecting the level and characteristics of CTCs, which might be helpful to find the most timely and optimized regimens.23 More importantly, the role of CTCs in lung tumor progression and metastasis depends both on obtaining sufficient numbers and the enumeration of CTCs for downstream assays.24 The potential role of CTCs for the early diagnosis of tumors, to assess prognosis, evaluate efficacy, develop targeted drugs, and individualize treatment is an issue of great importance.25 The results of previous studies have confirmed that the greater the number of CTCs detected in peripheral blood, the greater the risk of adverse prognosis in lung cancer patients.26 In this study, the value of FR‐positive CTC assays was used to diagnose lung adenocarcinoma. Consistent with previous studies, our ROC curve and Youden index indicated a useful cutoff value of 8.35 units.27 The diagnostic sensitivity and specificity of our sample of lung adenocarcinoma patients were 70.2% and 79.3%, respectively. To further analyze the efficacy of FR‐positive CTC detection in patients with different pathological types of lung adenocarcinoma, the sensitivity in patients with AIS, MIA, and invasive glands, and IA variants were 60%, 73.2%, 73.9%, and 75%, respectively. All results had satisfactory diagnostic validity (P < 0.05). The MTD was used as an indicator for tumor burden. Patients with lung cancer were divided into MTD ≤ 2 cm and >2 cm groups by MTD mean. Lung cancer patients with a heavy tumor burden had higher CTC levels. Measuring the CTC level and CEA could be a method to improve diagnostic efficacy for lung adenocarcinoma. In conclusion, detection of FR‐positive CTCs can be used as a potential molecular marker for adjuvant diagnosis and early detection of lung cancer in patients with lung adenocarcinoma. However, this study also has some limitations. As a single‐center sample study, there may be certain bias in the selected threshold of the subjects, thus a larger sample is required for further research. Acknowledgments The authors would like to thank all of the patients for their participation and cooperation. This work was supported in part by grants from the National Natural Science Foundation of China (8167110475) and Suzhou City's Science and Technology Plan Project (SYS201529). Disclosure No authors report any conflict of interest. References

|
Scooped by
Gilbert C FAURE
onto from Flow Cytometry to Cytomics September 13, 2018 3:43 AM
|
No comment yet.
Sign up to comment





 Your new post is loading...
Your new post is loading...