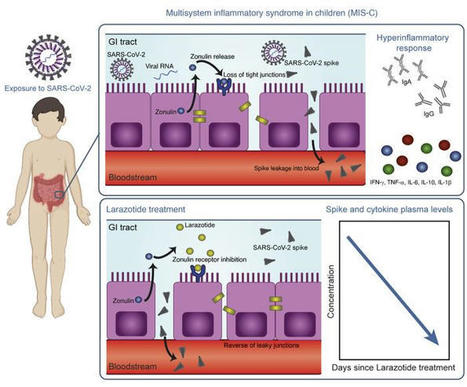
This study included biospecimens from 100 children: 19 children were clinically diagnosed with MIS-C as defined by CDC criteria (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI149633DS1); 26 children had COVID-19 confirmed by RT-PCR; and 55 children served as non–COVID-19 controls (32 pre-pandemic). The average age of the children with MIS-C (8 years old) was younger than that of the children who presented with COVID-19 (14 years old), which is consistent with the national averages (2, 15). The patients with MIS-C presented with a median of 3 days of acute symptoms associated with MIS-C (range, 1–28 days), after a previous COVID-19 exposure or SARS-CoV-2 infection 26 days prior to the presentation with MIS-C (range, 13–62 days). Notably, GI symptoms were predominant in the MIS-C cohort, affecting 89% of these patients, compared with 27% of children with acute COVID-19 (Fisher’s exact test, P < 0.0001; Table 1). Figure 1 outlines the specimens collected for the analysis and the timing of specimen collection from the children with MIS-C or acute COVID-19. Figure 1Study overview. Timing of sample collection and sample analysis for children with MIS-C or acute COVID-19. Table 1Age and sex for all pediatric patients and controls, and clinical features of illness for the children with acute COVID-19 or MIS-C SARS-CoV-2 in the GI tract of children with MIS-C coincides with a loss of intestinal epithelial barrier function. MIS-C develops several weeks after a SARS-CoV-2 infection or exposure (Table 1), and the viral load in respiratory secretions is known to decrease over the course of 7–10 days after infection (11, 16, 17). As most children with MIS-C have negative nasopharyngeal viral swabs (11), MIS-C is unlikely to be related to this initial infection of the respiratory tract. To assess the presence of SARS-CoV-2 in the GI tract, we measured SARS-CoV-2 RNA from MIS-C stool samples collected several weeks after the initial SARS-CoV-2 infection or exposure. Indeed, a majority of the patients showed detectable viral loads in the stool ranging from 1.5 × 102 to 2.5 × 107 RNA copies/mL, suggesting an ongoing nidus of infection in MIS-C (Supplemental Table 1). An intact, functional intestinal mucosal barrier should prevent the passage of large antigens from the gut lumen into the bloodstream, including viral antigens derived from SARS-CoV-2 present in the GI tract (12). Zonulin belongs to a family of structurally and functionally related proteins that reversibly regulate intestinal permeability by modulating intercellular tight junctions (18–20). Increased circulating zonulin levels resulting in increased intestinal permeability have been reported in several diseases (21) including autoimmune and hyperinflammatory diseases such as celiac disease (22), inflammatory bowel disease (23), and Kawasaki disease (24). Zonulin release from epithelial cells can result in permissibility of paracellular trafficking of large inflammatory antigens from the gut lumen into the bloodstream. In this study, mass spectrometry showed that, compared with controls, children with MIS-C had significantly increased release of zonulin into the circulation (P = 0.003; Figure 2A), which can result in a breakdown of mucosal barrier function. Correspondingly, children with MIS-C also had increased LPS-binding protein (LBP) levels compared with controls (P = 0.007; Figure 2B), signaling increased microbial translocation. Levels of soluble CD14, another marker of microbial translocation, were also increased in children with MIS-C (P = 0.1; Figure 2C), indicating a loss of GI mucosal barrier integrity. None of these markers of GI barrier integrity — zonulin, LBP, or CD14 — was significantly increased in the children with acute COVID-19 infection. Identification of SARS-CoV-2 within the stool, coupled with the loss of competency of tight junctions seen in MIS-C, but not acute COVID-19, suggests that GI sources of SARS-CoV-2 viral components could breach the mucosal barrier and enter the circulation. Figure 2Plasma (A) zonulin, (B) LPS-binding protein, and (C) soluble CD14 levels were quantified by multiplexed MS–based proteomics (54, 55) for children with MIS-C (n = 13) or COVID-19 (n = 21) and for non–COVID-19 control participants (n = 23). Results were compared by ANOVA. (D) SARS-CoV-2 spike, (E) S1, and (F) nucleocapsid protein levels were quantified in plasma from children with MIS-C (n = 16), children with acute COVID-19 (n = 22), and pre-pandemic healthy controls (n = 32). Results were compared by 1-way ANOVA with multiple comparisons. * P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Median values and 95% CI are presented. Children with MIS-C have SARS-CoV-2 antigenemia. Although viremia and antigenemia have been shown to correlate with severe acute COVID-19 in adults (17, 25), viremia has not been detected in MIS-C (11), and antigenemia has not previously been assessed in children. Using single-molecule array (Simoa) assays (25), we detected SARS-CoV-2 spike, S1, and nucleocapsid antigens in the plasma of children with MIS-C despite being weeks past their initial SARS-CoV-2 infection or exposure. The SARS-CoV-2 spike protein was significantly elevated in patients with MIS-C compared with healthy controls (P < 0.0001) and compared with children with acute COVID-19 (P < 0.001; Figure 2D). We also detected the SARS-CoV-2 S1 protein at significantly elevated levels in patients with MIS-C compared with healthy controls (P = 0.004) and with children with acute COVID-19 (P = 0.02; Figure 2E). SARS-CoV-2 nucleocapsid protein levels were increased in patients with MIS-C, although these levels did not reach significance (Figure 2F). We detected no significant increase in SARS-CoV-2 antigenemia in children with acute COVID-19 as compared with healthy controls. SARS-CoV-2 antigens showed no correlation with age (Supplemental Figure 1). Circulating SARS-CoV-2 antigen indicates that viral components have leaked from infected tissues. We attribute circulating antigen in patients with MIS-C to leakage from the GI system, as corroborated by the increased zonulin levels and microbial translocation markers we detected in the patients with MIS-C. The SARS-CoV-2 spike protein, specifically the S1 component (9, 10), has been hypothesized to have superantigen-like properties that are similar to those seen in the bacterial superantigen–mediated toxic shock syndrome. Superantigens bind to specific β-chains of T cell receptors (TCRs) at the variable domain in a complementary-determining region 3–independent (CDR3-independent) manner (26), thereby bypassing TCR specificity and resulting in skewing and overrepresentation of specific TCRs. Recent reports have identified TCR β variable (TRBV) gene skewing in MIS-C, with a profound expansion of TRBV11-2 (10, 27). Our data reveal a strong correlation between S1 antigenemia and the previously reported expression of TRBV11-2 in MIS-C (ref. 10; Pearson’s correlation, r = 0.89, P = 0.0005; Supplemental Figure 2). We found no correlation between the spike or nucleocapsid antigens and TRBV11-2 expression (Supplemental Figure 2). Consistent with hyperinflammatory responses, children with MIS-C experienced a cytokine storm with significantly elevated levels of IL-1β, IL-6, IL-10, and TNF-α (Supplemental Figure 3, A–D). Notably, the antiviral cytokine IFN-γ was also significantly increased in patients with MIS-C compared with both healthy controls and children with acute COVID-19 (Supplemental Figure 3E). This increase in IFN-γ is typical of a viral exposure, however, it can be paradoxically suppressed in adults with severe acute COVID-19 (28). We also assessed IL-12p70, IL-8, IL-5, and IL-22 levels but found that they were not altered in the children with MIS-C or acute COVID-19 (Supplemental Figure 3, F–I). MIS-C immunoprofiles reflect ongoing mucosal exposure to SARS-CoV-2. The immunoglobulin IgM, IgG, and IgA subsets targeting SARS-CoV-2 antigens in patients with MIS-C were most highly elevated against spike and S1 proteins (Figure 3, A–C, and Supplemental Figure 4), corresponding to the antigens most highly detected in the bloodstream of these children. As expected, anti–spike IgM was highest in children with acute COVID-19, reflecting early adaptive immune responses (Figure 3A). However, anti–spike IgM levels remained higher than would be expected, given that MIS-C presents weeks after the original infection with or exposure to SARS-CoV-2, but was on a downward trend over time (Figure 3D). As expected, anti–spike IgG, anti–S1 IgG, and anti–RBD IgG were highest in the children with delayed-onset MIS-C (Figure 3B and Supplemental Figure 4) and remained plateaued over time (Figure 3E). IgA is the immunoglobulin most reflective of mucosal immunity and typically wanes following viral clearance (29). Here, the levels of anti–spike IgA, anti–S1 IgA, and anti–RBD IgA were all significantly increased in the patients with MIS-C (Figure 3C and Supplemental Figure 4), and anti–spike IgA remained unexpectedly elevated for months after the initial SARS-CoV-2 infection (Figure 3F). The persistence of elevated anti–SARS-CoV-2 IgA and IgM in patients with MIS-C supports the hypothesis of ongoing viral antigenic exposure and inflammation in the GI mucosal surfaces of children with MIS-C. Figure 3Peak values of (A) anti–spike IgM, (B) anti–spike IgG, and (C) anti–spike IgA were quantified in plasma from children with MIS-C (n = 16), children with acute COVID-19 (n = 22), and pre-pandemic healthy controls (n = 32). Results were compared by 1-way ANOVA with multiple comparisons. Time course of (D) anti–spike IgM, (E) anti–spike IgG, and (F) anti–spike IgA were plotted over time following symptom onset for MIS-C. (G) The IC50 for antibody neutralization for children with MIS-C and children with acute COVID-19 were compared by Mann-Whitney t test. Mean values and the SD are presented. *P < 0.05, **P < 0.01, and ****P < 0.0001. We compared the capacity of plasma neutralization against SARS-CoV-2 between children with MIS-C and those with acute COVID-19. Interestingly, although the children with MIS-C had significantly elevated IgG antibody titers against SARS-CoV-2 relative to those with acute COVID-19, the neutralization titers were comparable between the children from both groups (Figure 3G). This suggests that in children with MIS-C, increasing quantities of IgG antibodies against the SARS-CoV-2 spike protein did not result in a gain in neutralizing capacity over time. Ineffective neutralization and poor antigen clearance, combined with ongoing viral antigen leakage from GI sources, could partially explain the high levels of antigen detected in the children with MIS-C. Temporal kinetics reveal that SARS-CoV-2 antigenemia is inadequately contained by humoral responses in MIS-C. Both SARS-CoV-2 antigen and immunoglobulins were detectable in patients with MIS-C at levels significantly higher than those detected in healthy controls. Antigenemia in adults occurs early in the COVID-19 disease course and is associated with pulmonary symptoms (25), whereas in MIS-C, antigenemia is found in the setting of prominent GI symptoms weeks to months after resolution of a SARS-CoV-2 upper respiratory tract infection or asymptomatic infection. To understand the relationship between viral antigenemia and the humoral response, we studied longitudinal samples from patients with MIS-C. First, we assessed antigens over time following MIS-C symptom onset. Previous reports show that in adults with acute COVID-19, SARS-CoV-2 antigens are rapidly cleared as the patient reaches seroconversion (25, 30). In contrast, our studies showed that spike antigens rose over the first few days of MIS-C symptoms and persisted for more than 10 days, occasionally through 6 months (Supplemental Figure 5), despite seroconversion to anti–spike IgG and anti–spike IgA antibodies. The elevated presence of spike protein in seroconverted patients was not observed in any adult COVID-19 cases (25). We also found that SARS-CoV-2 antigen levels did not significantly decrease in our cohort following the initiation of steroid and/or intravenous immunoglobulin (IVIG) replacement therapy (Figure 4A), which are the only currently recommended treatments for MIS-C (31). This suggests that current therapies are targeted toward the downstream consequences of MIS-C, namely the inflammatory responses, but fail to address the ongoing antigenemia instigating ongoing inflammation. Figure 4B provides a detailed overview of spike antigenemia and the humoral inflammatory responses in a representative hospitalized child with MIS-C, relative to SARS-CoV-2 exposure, symptom onset, and treatment course. Figure 4(A) SARS-CoV-2 spike, S1, and nucleocapsid levels in plasma from children with MIS-C were quantified before treatment with steroids and/or immunoglobulin replacement therapy, through 14 days following treatment (n = 11). Shaded regions signify the limit of detection for each specific antigen test. (B) Spike and anti–spike IgM, anti–spike IgG, and anti–spike IgA levels were measured over the course of illness of a child with MIS-C. Of note, the spike protein remained above the limit of detection for the spike antigen test at the 213-day follow-up point. Zonulin antagonism reduces spike antigenemia and cytokine storm, with subsequent improvement in clinical outcomes in MIS-C. Although steroids and IVIG do not block leakage of SARS-CoV-2 antigen across the mucosal barrier, therapies targeting GI mucosal permeability could potentially reduce or prevent antigenemia. Larazotide, a zonulin antagonist, is an investigational therapy that has been well characterized in preclinical trials (32, 33), with an excellent safety profile (34), and is currently in phase III trials for the treatment of refractory celiac disease (35). Given this theoretical benefit, we obtained US FDA approval for compassionate use of larazotide, 10 μg/kg every 6 hours, to treat a critically ill 17-month-old boy with MIS-C after he failed to improve with antiinflammatory therapies. The toddler, who had a complex past medical history including partial duplication of chromosome 14, biliary atresia status post Kasai procedure, and gastrotomy tube placement, with frequent episodes of ascending cholangitis, required hospitalization for severe COVID-19, complicated by respiratory failure and cardiac resuscitation. One month after he was diagnosed with COVID-19, he developed abdominal compartment syndrome with a C-reactive protein (CRP) level of 286 mg/L, a ferritin level of 51,223 μg/L, and a N-terminal pro-b–type natriuretic peptide (NT-proBNP) level of 16,462 pg/mL without evidence of cardiac injury on echocardiogram. He was treated with IVIG, steroids, and anakinra with transient improvement of inflammatory markers but rapid recrudescence of symptoms. We detected 1020 copies of SARS-CoV-2 RNA in the patient’s stool, as quantified by RT-PCR, and a plasma spike antigen level of 566 pg/mL more than 2 weeks after initiation of IVIG and steroid treatment for MIS-C. After the initiation of larazotide, his CRP level, which had quickly rebounded to 173 mg/L following withdrawal of anakinra, dropped by 85% from peak values, and spike antigen levels dropped by 90% to 59 pg/mL (Figure 5A). The SARS-CoV-2 nucleocapsid protein levels also dropped by 98% from 77 pg/mL to 1.45 pg/mL (limit of detection). His fever curve, which also rebounded after discontinuation of anakinra, improved, as did his ferritin and D-dimer levels (Figure 5A). The patient also experienced an improvement in cytokine and inflammatory mediator levels, including IL-1β, IFN-γ, IL-2R, and IL-17, representing an improvement in inflammasome activation, virus-induced IFN release, and immune cell activation (Figure 5B). These cytokines and inflammatory mediators initially spiked during the development of MIS-C, then improved following IVIG but plateaued or rebounded despite steroid treatment. We observed an improvement in these inflammatory mediators following the precipitous drop in SARS-CoV-2 antigens, which occurred as a result of or coincident with the initiation of larazotide therapy. Importantly, the patient achieved his longest stretches without fever since his hospital admission, his GI symptoms improved and he was able to resume full feedings, and his ventilatory status improved. Figure 5Timeline for the child treated with larazotide. The hospital course is delineated by hospitalization for acute COVID-19 (day 0) followed by development of MIS-C (day 39), with the treatment courses identified along the time course. (A) CRP and SARS-CoV-2 spike antigen levels, the median daily temperature (temp) curve, and D-dimer and ferritin levels throughout the hospital course are shown. Light blue shading represents a normal body temperature. The light gray shading highlights the days of larazotide treatment. (B) Inflammatory cytokine IL-17F, IL-2R, IFN-γ, and IL-1β levels following the development of MIS-C, in relation to the treatment courses.

|
Scooped by
Gilbert C FAURE
onto Mucosal Immunity March 29, 2022 3:37 AM
|
No comment yet.
Sign up to comment




 Your new post is loading...
Your new post is loading...