Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
June 29, 2025 7:30 AM
|
A review of currently licensed mucosal COVID-19 vaccines
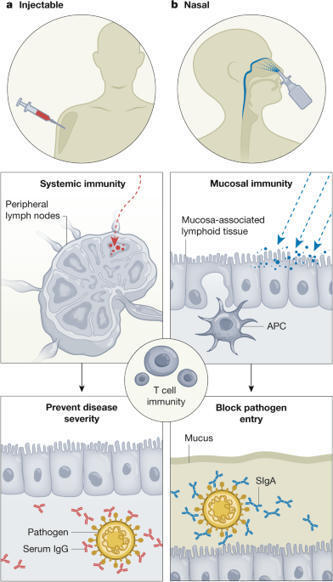
Beginning with Edward Jenner’s discovery of the smallpox vaccine, the ever-expanding repertoire of vaccines against pathogens has saved many lives. During the COVID-19 pandemic, a revolutionary mRNA injectable vaccine emerged that effectively controlled the severity of disease caused by SARS-CoV-2. This vaccine induced potent antigen-specific neutralizing serum IgG antibodies, but was limited in its ability to prevent viral invasion at the respiratory surfaces. Nasal vaccines have attracted attention as a potential strategy to combat respiratory infections and prepare for future pandemics. Input from disciplines such as microbiology, biomaterials, bioengineering and chemistry have complemented the immunology to create innovative delivery systems. This approach to vaccine delivery has yielded nasal vaccines that induce secretory IgA as well as serum IgG antibodies, which are expected to prevent pathogen invasion, thereby diminishing transmission and disease severity. For a nasal vaccine to be successful, the complexity of the relevant anatomical, physiological and immunological properties, including the proximity of the central nervous system to the nasal cavity, must be considered. In this Review, we discuss past and current efforts as well as future directions for developing safe and effective nasal vaccines for the prevention of respiratory infections. This Review provides an overview of progress and future directions in the development of nasally administered vaccines for respiratory infections.

|
Scooped by
Gilbert C FAURE
April 13, 2025 4:12 AM
|

|
Scooped by
Gilbert C FAURE
January 21, 2025 12:15 PM
|

|
Scooped by
Gilbert C FAURE
June 3, 2024 11:33 AM
|
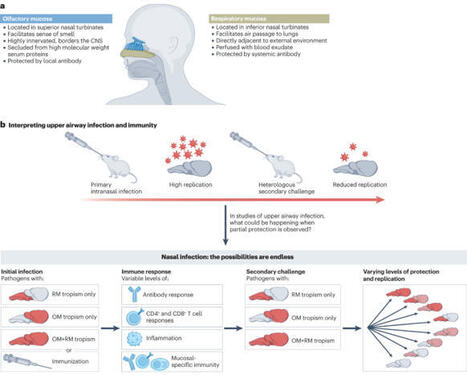
The olfactory mucosa is a component of the nasal airway that mediates the sense of smell. Recent studies point to an important role for the olfactory mucosa as a barrier to both respiratory pathogens and to neuroinvasive pathogens that hijack the olfactory nerve and invade the CNS. In particular, the COVID-19 pandemic has demonstrated that the olfactory mucosa is an integral part of a heterogeneous nasal mucosal barrier critical to upper airway immunity. However, our insufficient knowledge of olfactory mucosal immunity hinders attempts to protect this tissue from infection and other diseases. This Review summarizes the state of olfactory immunology by highlighting the unique immunologically relevant anatomy of the olfactory mucosa, describing what is known of olfactory immune cells, and considering the impact of common infectious diseases and inflammatory disorders at this site. We will offer our perspective on the future of the field and the many unresolved questions pertaining to olfactory immunity. This Review from Wellford and Moseman considers the unique immunology of the olfactory mucosa. The authors describe how stromal cells, innate immune cells and adaptive immune cells cooperate to defend the olfactory mucosa, protecting the host from potentially serious respiratory or neurotropic infections. They also discuss the relevance of olfactory mucosal immunology for the fields of vaccination and neurodegeneration.

|
Scooped by
Gilbert C FAURE
April 18, 2024 4:30 AM
|
Abstract. Established evidence indicates that oral microbiota plays a crucial role in modulating host immune responses to viral infection. Following severe

|
Scooped by
Gilbert C FAURE
March 7, 2024 11:28 AM
|
The potential of COVID-19 booster vaccine products (antibodies) passed from mother to offspring through breastmilk.

|
Scooped by
Gilbert C FAURE
January 11, 2024 6:16 AM
|
Researchers have developed a novel intranasal COVID-19 vaccine that enhances the immune system’s response to the virus, providing longer-lasting, greater protection than vaccine injections, even against new and emerging variants.

|
Scooped by
Gilbert C FAURE
December 15, 2023 11:22 AM
|

|
Scooped by
Gilbert C FAURE
December 8, 2023 11:17 AM
|

|
Scooped by
Gilbert C FAURE
November 30, 2023 4:43 AM
|
When the COVID-19 pandemic first started, no effective anti-viral drugs were available to fight the disease. However, in record time, so-called monoclonal antibodies were developed as a lifesaving treatment.

|
Rescooped by
Gilbert C FAURE
from Virus World
November 6, 2023 3:40 AM
|
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA generally becomes undetectable in upper airways after a few days or weeks postinfection. Here we used a model of viral infection in macaques to address whether SARS-CoV-2 persists in the body and which mechanisms regulate its persistence. Replication-competent virus was detected in bronchioalveolar lavage (BAL) macrophages beyond 6 months postinfection. Viral propagation in BAL macrophages occurred from cell to cell and was inhibited by interferon-γ (IFN-γ). IFN-γ production was strongest in BAL NKG2r+CD8+ T cells and NKG2Alo natural killer (NK) cells and was further increased in NKG2Alo NK cells after spike protein stimulation. However, IFN-γ production was impaired in NK cells from macaques with persisting virus. Moreover, IFN-γ also enhanced the expression of major histocompatibility complex (MHC)-E on BAL macrophages, possibly inhibiting NK cell-mediated killing. Macaques with less persisting virus mounted adaptive NK cells that escaped the MHC-E-dependent inhibition. Our findings reveal an interplay between NK cells and macrophages that regulated SARS-CoV-2 persistence in macrophages and was mediated by IFN-γ. Huot et al. show that interferon-γ (IFN-γ) regulates the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in bronchoalveolar macrophages from cynomolgus macaques up to 18 months postinfection. Published in Nat. Immunology (Nov. 2, 2023): https://doi.org/10.1038/s41590-023-01661-4
Via Juan Lama

|
Suggested by
LIGHTING
August 19, 2023 5:11 AM
|
One of the most preferable characteristics for a COVID-19 vaccine candidate is the ability to reduce transmission and infection of SARS-CoV-2, in addition to disease prevention. Unlike intramuscular vaccines, intranasal COVID-19 vaccines may offer this by generating mucosal immunity. In this open-label, randomised, multicentre, phase 3 clinical trial (CTRI/2022/02/40065; ClinicalTrials.gov: NCT05522335), healthy adults were randomised to receive two doses, 28 days apart, of either intranasal adenoviral vectored SARS-CoV-2 vaccine (BBV154) or licensed intramuscular vaccine, Covaxin®. Between April 16 and June 4, 2022, we enrolled 3160 subjects of whom, 2971 received 2 doses of BBV154 and 161 received Covaxin. On Day 42, 14 days after the second dose, BBV154 induced significant serum neutralization antibody titers against the ancestral (Wuhan) virus, which met the pre-defined superiority criterion for BBV154 over Covaxin®. Further, both vaccines showed cross protection against Omicron BA.5 variant. Salivary IgA titers were found to be higher in BBV154. In addition, extensive evaluation of T cell immunity revealed comparable responses in both cohorts due to prior infection. However, BBV154 showed significantly more ancestral specific IgA-secreting plasmablasts, post vaccination, whereas Covaxin recipients showed significant Omicron specific IgA-secreting plasmablasts only at day 42. Both vaccines were well tolerated. Overall reported solicited reactions were 6.9% and 25.5% and unsolicited reactions were 1.2% and 3.1% in BBV154 and Covaxin® participants respectively.
|

|
Scooped by
Gilbert C FAURE
June 8, 2025 1:53 PM
|
A Review of Currently Licensed Mucosal COVID-19 Vaccines -
Recent review on mucosal COVID-19 vaccines approved by the FDA. Unlike most injectable vaccines, they induce a robust mucosal immune response with secretory IgA antibodies. Mucosal vaccines also lead to a strong systemic cellular and humoral immune response.
To date, only five active mucosal vaccines have received regulatory approval for human use, and at least 29 are under clinical trials. Approved vaccines use intranasal, sometimes also combined with intramuscular doses. Most of them utilize replication incompetent viruses (e.g. adenovirus) to deliver the antigen to the nasal mucose via nasal sprays or nebulizers.
https://sco.lt/8DN1Jg
#covid19 #health #globalhealth #publichealth #medicine #biotechnology #pharmaceuticals #FDA #WHO #CDC #ECDC #NIAID #clinicaltrials #immunology #vaccines

|
Scooped by
Gilbert C FAURE
May 5, 2025 5:02 AM
|
🌍 Le futur des vaccins : des solutions nasales contre les infections respiratoires 🦠
Dans le cadre de la Semaine Européenne de la Vaccination, découvrez les dernières innovations dans le domaine des vaccins, avec un focus particulier sur les vaccins nasaux permettant une protection plus efficace et ciblée. 👃
🚀 Ces solutions novatrices sont développées pour protéger les générations futures contre des infections respiratoires graves telles que la coqueluche, la pneumonie à pneumocoques, et les virus comme la #grippe et le Covid-19. 🫁
Des chercheurs européens, dont l’Institut Pasteur de Lille, sont à la pointe de ces découvertes avec des projets comme :
🟢 BPZE1 : Un vaccin nasal contre la coqueluche en développement, il bloque la transmission et offre une protection durable, avec des résultats positifs chez les enfants et adultes ! 🎉
🟢 Vaccin nasal contre les pneumocoques : Ce vaccin protège contre toutes les souches de pneumocoques, une avancée importante pour protéger les plus vulnérables ! 👶👵
👉 Pour en savoir plus, rendez-vous sur pasteur-lille.fr

|
Rescooped by
Gilbert C FAURE
from Virus World
January 29, 2025 1:26 PM
|
The “Ferrari of viruses” is having a banner season. Norovirus, which races through cruise ships, homes, and long-term care facilities, is experiencing a remarkable winter surge in the Northern Hemisphere, sending large numbers of people racing to the bathroom and many others to the hospital, and in rare cases, proving fatal. In the United States, for example, 91 outbreaks of the intestinal virus occurred in the first week of December 2024, far above the previous maximum, 65, for the same week between 2010 and 2024. And levels of its genes in U.S. wastewater are an order of magnitude above last year. “The early data for the early part of the season is certainly supporting that we’re going to have a pretty intense norovirus year,” says Lisa Lindesmith, who studies the virus at the University of North Carolina (UNC) at Chapel Hill. Some of the surge may be due to a new variant of the virus, unfamiliar to many people’s immune systems, and the resumption of cruises and other gatherings that the COVID-19 pandemic interrupted. And there’s no vaccine anywhere in sight: The most advanced candidate just failed a key trial and others won’t be ready for several years. Norovirus thrives in cold climes, causing explosive diarrhea and vomiting that typically only last for a day. But several weeks after people recover, they can still shed the virus, and it can remain infectious for long periods on surfaces. It’s notoriously resistant to many disinfectants, and studies in adult volunteers have shown just a trace of virus is enough to sicken a person. Oysters are also a source of infection, because the filter-feeding mollusks concentrate the virus from contaminated water in their tissues. U.S. health officials issued several warnings about infected oysters in December, and France has banned oyster harvesting in certain regions because of norovirus outbreaks. For most people the virus is a rapidly passing misery that creates the wrong kind of unforgettable memories from a wedding or a holiday gathering. But, “It can be pretty severe,” says Mary Estes, a virologist at the Baylor College of Medicine who is a leading researcher in this small field. Although norovirus-related mortality is low in wealthy countries, it kills an estimated 200,000 young children in the developing world each year. Even in the U.S., it’s the leading cause of hospitalization for diarrheal disease, hitting young children and the elderly particularly hard. Immunocompromised people, particularly cancer patients on chemotherapy or transplant recipients taking antirejection drugs, can have chronic infections that last for months or even years. Many sickened people also miss work, globally leading to an estimated $60 billion in annual losses. Given that symptoms quickly resolve in most cases, few people seek care, but in the U.S., reports of norovirus-related outbreaks—two or more illnesses from a common source—this winter promise to far outstrip the 2500 typically seen. Cruise ships are being hit hard, too. In the United Kingdom, labs that test for the virus reported 63% more positive samples in the last 2 weeks of 2024 than the average, and hospitalizations were up 11%. Albert Kapikian at the U.S. National Institutes of Health discovered the first norovirus in 1972 from an outbreak of “winter vomiting disease” that occurred at an elementary school in Norwalk, Ohio—hence the “noro” in the name. Scientists have since described more than 30 major variants that circulate in humans, distinguished by changes in their surface protein, VP1, which is the main target of antibodies that can “neutralize” it. “This virus is really good at getting around antibodies,” says veteran norovirus researcher Christine Moe of Emory University. “I used to think it was the most successful pathogen until COVID came along.” The world had four massive waves of norovirus between 2002 and 2012—“the most pandemics of any virus in the 21st century,” says UNC’s Ralph Baric, who has long studied the pathogen. Each erupted when a new norovirus variant replaced the dominant one. But since 2012, a set of related variants called GII.4 has held sway. “One of the hottest topics in norovirus research is why, after having those really frequent replacement events, has that process slowed down?” says Lindesmith, who works in Baric’s lab. A change that could help explain this winter’s surge in cases is the success of GII.17, a variant that has circulated at low levels for decades. GII.17 began to rise in the U.S. and six European countries last year, a 26 September 2024 paper in Eurosurveillance reported. According to the U.S. Centers for Disease Control and Prevention, 70% of 56 viral isolates sequenced between 1 September and the end of the year belong to the GII.17 lineage. The U.K. and the Netherlands are also seeing GII.17 dominate. Whether it will end GII.4’s 12-year reign as the dominant variant won’t be clear for many months. What finally gave GII.17 an edge isn’t clear. Norovirus research Miranda de Graaf at the Erasmus Medical Center says the virus found in the Netherlands has significantly mutated away from other GII.17 strains. The drop in norovirus transmission because of social distancing during the COVID-19 pandemic may also have played a role. “If you go 3 or 4 years without much exposure in the population, the prediction would be that that background immunity is going to drop over time, and then there’s going to be a big surge that occurs sometime after as things return to normal,” Baric says. A norovirus vaccine likely would have a large global market among all ages, but development was slow off the mark. “It took a long time for people to recognize that these viruses really are infecting a lot of people,” Estes says, which limited funding for the research. The virus is also a challenge to work with; it can only grow reliably in a complicated organoid culture system, developed by Estes and co-workers, that mimics human intestines. The most advanced vaccine candidate, which contained “viruslike particles” made of VP1, failed in an efficacy trial in infants, its manufacture, HilleVax, reported in July 2024. A Chinese company, Zhifei, has an efficacy trial underway in children of a candidate that contains VP1s from four genotypes, including GII.4 and GII.17. And Moderna in September 2024 launched an efficacy trial in adults of a vaccine that uses messenger RNAs encoding VP1s from multiple variants (although not GII.17). Even if a norovirus vaccine proves safe and effective, it is up against what Baric calls “one of the most infectious viruses in nature,” which means a vaccine is unlikely to prevent all symptoms and completely stop transmission. It would probably also require regular boosters. “This is not going to be a one-and-done sort of vaccination,” Lindesmith says. But like COVID-19 vaccines, a norovirus vaccine might slow spread and spare people from severe disease or even death—keeping one day of misery from turning into something much worse. doi: 10.1126/science.zapmdgv
Via Juan Lama

|
Scooped by
Gilbert C FAURE
August 2, 2024 4:15 AM
|
The findings, published July 31 in Science Advances, provide further evidence that so-called mucosal vaccines sprayed into the nose or dropped into the mouth…

|
Scooped by
Gilbert C FAURE
June 2, 2024 5:34 AM
|
Currently, SARS-CoV-2 has evolved into various variants, including the numerous highly mutated Omicron sub-lineages, significantly increasing immune evasion ability. The development raises concerns about the possibly diminished effectiveness of available vaccines and antibody-based therapeutics.

|
Scooped by
Gilbert C FAURE
March 21, 2024 8:30 AM
|
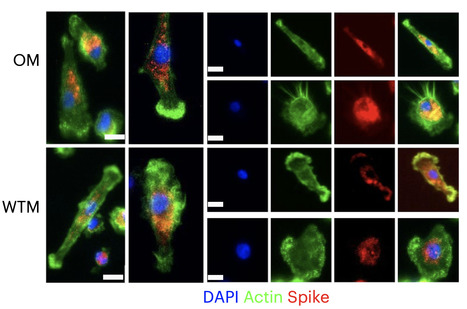
SPRAY NASAL PARA ENCERRAR A PANDEMIA COMPLETAMENTE
Uma vacina que, além de proteger contra a Covid-19, acabaria com a transmissão do vírus SARS-CoV-2.
Esse é…

|
Scooped by
Gilbert C FAURE
February 4, 2024 2:39 AM
|
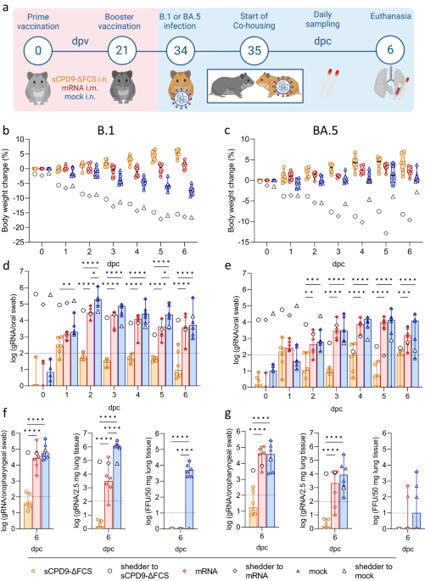
The development of effective SARS-CoV-2 vaccines has been essential to control COVID-19, but significant challenges remain. One problem is intramuscular administration, which does not induce robust mucosal immune responses in the upper airways—the primary site of infection and virus shedding. Here we compare the efficacy of a mucosal, replication-competent yet fully attenuated virus vaccine, sCPD9-ΔFCS, and the monovalent mRNA vaccine BNT162b2 in preventing transmission of SARS-CoV-2 variants B.1 and Omicron BA.5 in two scenarios. Firstly, we assessed the protective efficacy of the vaccines by exposing vaccinated male Syrian hamsters to infected counterparts. Secondly, we evaluated transmission of the challenge virus from vaccinated and subsequently challenged male hamsters to naïve contacts. Our findings demonstrate that the live-attenuated vaccine (LAV) sCPD9-ΔFCS significantly outperformed the mRNA vaccine in preventing virus transmission in both scenarios. Our results provide evidence for the advantages of locally administered LAVs over intramuscularly administered mRNA vaccines in preventing infection and reducing virus transmission. In this study, the authors evaluated the protective capacity of a mucosal, live-attenuated SARS-CoV-2 vaccine and show that it induces systemic and mucosal humoral immunity, protects from clinical disease symptoms, and prevents virus transmission in hamsters more efficiently than an intramuscular mRNA vaccine.

|
Scooped by
Gilbert C FAURE
January 11, 2024 6:15 AM
|
Researchers at Karolinska Institutet have shown that nasal drops with IgA antibodies can protect mice from SARS-CoV-2 infection. The results imply a new way to protect individuals at high risk from different variants of the SARS-CoV-2 virus and possibly other infections. The study is published in PNAS.

|
Scooped by
Gilbert C FAURE
December 14, 2023 2:38 AM
|

|
Scooped by
Gilbert C FAURE
December 2, 2023 3:02 AM
|
Exhaled SARS-CoV-2-containing aerosols contributed significantly to the rapid and vast spread of covid-19. However, quantitative experimental data on the infectivity of such aerosols is missing. Here, we quantified emission rates of infectious viruses in exhaled aerosol from individuals within...

|
Scooped by
Gilbert C FAURE
November 30, 2023 4:42 AM
|
Why are severe courses of SARS-CoV-2 infection much less common in children and adolescents than in adults?Scientists at the German Cancer Research Center have now discovered that the immune system in the upper respiratory tract is much more alert and active in children before infection than in adu...

|
Scooped by
Gilbert C FAURE
October 12, 2023 3:57 AM
|
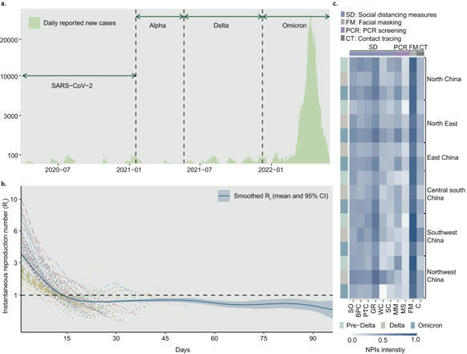
Targeted public health interventions for an emerging epidemic are essential for preventing pandemics. During 2020-2022, China invested significant efforts in strict zero-COVID measures to contain outbreaks of varying scales caused by different SARS-CoV-2 variants. Based on a multi-year empirical dataset containing 131 outbreaks observed in China from April 2020 to May 2022 and simulated scenarios, we ranked the relative intervention effectiveness by their reduction in instantaneous reproduction number. We found that, overall, social distancing measures (38% reduction, 95% prediction interval 31-45%), face masks (30%, 17-42%) and close contact tracing (28%, 24-31%) were most effective. Contact tracing was crucial in containing outbreaks during the initial phases, while social distancing measures became increasingly prominent as the spread persisted. In addition, infections with higher transmissibility and a shorter latent period posed more challenges for these measures. Our findings provide quantitative evidence on the effects of public-health measures for zeroing out emerging contagions in different contexts. China maintained a ‘zero-COVID’ policy from early in the pandemic until late 2022 that employed various public health interventions with the aim of COVID-19 containment. Here, the authors use data from 131 outbreaks in China to estimate the effects of a range of interventions against different SARS-CoV-2 variants in diverse settings.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...












![[Video] CT Vacinas | Centro de Tecnologia de Vacinas on LinkedIn: #spraynasal #vacinaintranasal #usp #jorgekalil #ctvacinas | Mucosal Immunity | Scoop.it](https://img.scoop.it/EET5qrxV8MsF79LoVoTSrDl72eJkfbmt4t8yenImKBVvK0kTmF0xjctABnaLJIm9)