Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
December 27, 2013 10:35 AM
|
is the most recent part of Immunology! It appeared less than 40 years ago, while systemic immunity exploded 60 years ago. It is still a minor part of Immunology teaching and research, while the mucosal immune system is at the frontline of encounters with germs, antigens... in other words the environment. major keywords: > 450 posts IgA http://www.scoop.it/t/mucosal-immunity?q=IgA > 125 posts tolerance http://www.scoop.it/t/mucosal-immunity?q=tolerance > 400 posts : microbiome http://www.scoop.it/t/mucosal-immunity?q=microbiome july 2015: almost 2100 scoops, >1700 visitors, >3900 views november 2017 >10K views of >3300 scoops june 2020 >17.6K views, >5.5K visitors, >4.5K scoops may 2024 >22K views, >6.9 visitors, >5.2 scoops

|
Scooped by
Gilbert C FAURE
February 20, 3:00 AM
|
Nasal spray vaccine could ‘replace multiple jabs every year’Bookmark popoverRemoved from bookmarksClose popoverScientists at Stanford Medicine have developed a universal vaccine formula, tested on mice, that offers broad protection against various respiratory threats. The vaccine, delivered as a nasal spray, could protect against cold, flu, Covid, allergies, respiratory viruses, sepsis-causing bacteria, and even house dust mites. It works by mimicking the signals immune cells use to communicate during an infection, rather than targeting specific parts of a pathogen. If developed for humans, this vaccine could replace multiple annual jabs for winter respiratory infections and potentially protect against new pandemic bugs. While lead author Dr Bali Pulendran estimates human availability within five to seven years, other experts caution that a truly universal vaccine is still some way off due to safety considerations and the diversity of the human population.

|
Scooped by
Gilbert C FAURE
February 16, 6:27 AM
|
Une avancée importante en recherche vaccinale pour les 6 mois à 5 ans
Une équipe dirigée par Guy Boivin, professeur au Département de pédiatrie et chercheur au Centre de recherche du CHU de Québec – Université Laval, a développé un vaccin expérimental administré par voie intranasale afin de protéger les jeunes enfants contre deux virus respiratoires majeurs : le métapneumovirus humain et le virus respiratoire syncytial (VRS). Ces deux agents infectieux sont responsables chaque année de nombreuses bronchiolites et pneumonies chez les jeunes enfants.
Les premières études menées sur des modèles animaux montrent des résultats très encourageants.
Cette avancée repose sur une plateforme vaccinale qui permet d’intégrer rapidement des éléments de différents virus pour créer de nouveaux candidats vaccins.
Découvrez tous les détails :

|
Scooped by
Gilbert C FAURE
February 10, 4:48 AM
|

|
Scooped by
Gilbert C FAURE
February 4, 6:39 AM
|
Ever had a client ask: "Why does my kitten need to come TWO times for vaccines? Can't we just do it all at once?"
Let me break down the science in a way that might change how it act
🧬 The "Goldilocks Problem" of Maternal Immunity
Kittens are born with almost NO immunity from their mother during pregnancy. Unlike humans, cats have a special type of placenta that blocks antibody transfer before birth. Instead, 90-95% of protective antibodies come through colostrum in those critical first 16 hours of life (Claus et al., 2006).¹
But here's where it gets tricky...
These maternal antibodies are both a blessing and a curse:
✅ They protect vulnerable kittens from deadly diseases
❌ But they ALSO attack vaccine antigens, preventing the kitten from building their own immunity
This creates what scientists call the "window of susceptibility", a period where kittens are:
-Too vulnerable to fight off real infections
-Yet unable to respond to vaccines
Consider these exposure risks for "indoor-only" cats:
-Panleukopenia virus survives for YEARS in the environment and can be tracked indoors on shoes and clothing
-Multi-cat households where ONE cat goes outside creates risk for ALL cats
Here's what evidence-based feline vaccination looks like in #2026:
For Kittens: → Start at 6-8 weeks, continue every 2-4 weeks until 16-20 weeks → Core vaccines: FPV, FHV-1, FCV → FeLV for ALL kittens (remember that age-resistance curve!) → Rabies at 12-16 weeks → yearly booster
For Adult High-Risk Cats: → Annual booster of Core and Rabies
What challenges do you face while Vaccination?
#mianpetsandvets #VeterinaryMedicine #FelineHealth #VetMed #CatVaccination #VeterinaryEducation #CatsOfLinkedIn

|
Scooped by
Gilbert C FAURE
January 24, 7:33 AM
|
Sign in or join now to see posts like this one and more.

|
Scooped by
Gilbert C FAURE
January 12, 9:07 AM
|
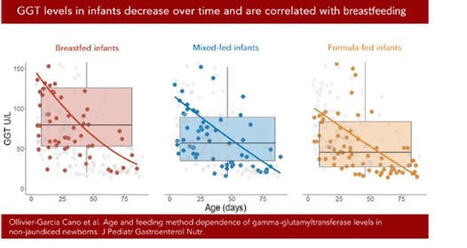
Une belle façon de démarrer 2026 ! Notre étude sur les taux de gamma-GT sanguins chez les nourrissons allaités vient d’être publiée dans le Journal of Pediatric Gastroenterology and Nutrition (JPGN). Ce travail, issu de la thèse d’Audrey Ollivier-Garcia Cano, a été mené en collaboration avec Marion Marlinge, Paul Guerry et Aurélie MORAND .
En médecine vétérinaire, le taux de gamma-GT est utilisé comme marqueur du transfert d’immunoglobulines via le colostrum chez les bovins (les IgG ne passant pas la barrière placentaire). Certaines études suggéraient par ailleurs que le lait maternel est riche en gamma-GT. Nous avons donc mené une étude rétrospective chez des nourrissons ayant eu un bilan hépatique et consultant aux urgences pédiatriques, en excluant ceux présentant une infection ou un ictère.
Les gamma-GT sériques étaient significativement plus élevées chez les enfants allaités (101 UI/l) que chez les non-allaités (64 UI/l), avec un niveau intermédiaire (77 UI/l) pour l’allaitement mixte. Nous avons également confirmé la diminution des gamma-GT avec l’âge.
Ces résultats soulignent l’importance d’interpréter les dosages de gamma-GT en fonction du mode d’allaitement. Ils rappellent aussi la richesse des approches pluridisciplinaires.
https://lnkd.in/dEnBgETK

|
Scooped by
Gilbert C FAURE
January 8, 1:43 PM
|
The gut virome is a complex ecosystem characterized by the interplay of diverse viral entities, predominantly bacteriophages and eukaryotic viruses. The gut virome has a critical role in human health by shaping microbial community profiles, modulating host immunity and influencing metabolic processes. Different viral metagenomics approaches have revealed the remarkable diversity of the gut virome, showing individual-specific patterns that evolve over time and adapt dynamically to environmental factors. Perturbations in this community are increasingly associated with chronic immune and inflammatory conditions, metabolic disorders and neurological conditions, highlighting its potential as a diagnostic biomarker and therapeutic target. The early-life gut virome is particularly influential in establishing lifelong health trajectories through its interactions with diet, immune pathways and others, thereby contributing to inflammatory and metabolic regulation. This Review synthesizes current knowledge of gut virome composition, dynamics and functional relevance, critically evaluating evidence distinguishing causal from correlative roles in disease pathogenesis. The interactions of the virome with other microbiome components and host immunity are examined, and emerging translational applications, including phage therapy and biomarker development, are discussed. Integrating these insights while acknowledging methodological challenges provides a comprehensive framework for understanding the complex roles of the gut virome in health and disease. The gut virome is a complex ecosystem and has a critical role in human health. This Review outlines gut virome composition and functional relevance, and its role in human health and disease. Methodological challenges in advancing our knowledge of the gut virome are also discussed.

|
Scooped by
Gilbert C FAURE
January 5, 4:21 AM
|
IgA friends at mucossl level and foes at systemic level… a new proof…

|
Scooped by
Gilbert C FAURE
January 2, 5:14 AM
|
I thought a banana was loaded with chemicals.
That’s until I found the ingredient list for human breast milk.
If you saw this list on a label, you might think it’s never been anywhere near a human.
And if you go by how they’re pronounced— you might be ready to starve an infant.
Good thing we don’t have anyone silly enough to believe that these chemicals are a bad thing…
——-
HUMAN MILK INGREDIENT LIST: Water, lactose, triacylglycerols [oleic acid, palmitic acid, linoleic acid, alpha-linolenic acid, stearic acid, lauric acid, myristic acid], phospholipids [sphingomyelin, phosphatidylcholine], cholesterol, free fatty acids [docosahexaenoic acid (DHA), arachidonic acid (ARA)]),
Oligosaccharides (2′-fucosyllactose, lacto-N-tetraose, lacto-N-neotetraose, 3′-sialyllactose, 6′-sialyllactose, fucosylated and sialylated oligosaccharides),
Milk Proteins (α-lactalbumin, lactoferrin, secretory immunoglobulin A, serum albumin, lysozyme, β-casein),
Minerals (potassium, calcium, chloride, phosphorus, sodium, magnesium, iron, zinc, iodine, copper, selenium, manganese),
Free Amino Acids (glutamic acid, glutamine, taurine, alanine, glycine, serine, threonine, valine, leucine, isoleucine, lysine, methionine, phenylalanine, tyrosine, tryptophan, cysteine, histidine),
Vitamins (vitamin A [retinol], vitamin D, vitamin E [α-tocopherol], vitamin K, vitamin C [ascorbic acid], thiamin [B1], riboflavin [B2], niacin [B3], pantothenic acid [B5], vitamin B6, folate [B9], vitamin B12),
Enzymes (bile salt–stimulated lipase, amylase, proteases),
Proprietary Blend (nucleotides, choline, phosphocholine, carnitine, inositol, polyamines),
Hormones & Growth Factor Blend (insulin, leptin, adiponectin, epidermal growth factor, insulin-like growth factor-1, transforming growth factor-β),
Cytokines & Immune Factor Blend (MicroRNAs, Living Cells (leukocytes, epithelial cells, stem-like cells)).
—-
#mammals #foodchemistry #foodscience #humanchemicalfactories #sciencerules | 80 comments on LinkedIn

|
Scooped by
Gilbert C FAURE
December 29, 2025 6:08 AM
|
Efficacy, Immunogenicity, and Safety of the Live-Attenuated Intranasal Pertussis Vaccine BPZE1: A Randomised, Placebo-Controlled Phase 2b Human Challenge Study in the UK:
The resurgence of pertussis is largely attributed to suboptimal vaccination coverage, particularly in countries that rely exclusively on acellular vaccines, which fail to induce mucosal immunity and generate minimal indirect (herd) protection. Consequently, sustained coverage levels above 95% are required to control transmission. BPZE1 is a live-attenuated Bordetella pertussis strain developed for intranasal administration, engineered through the genetic inactivation or deletion of three key virulence factors—pertussis toxin (PT), dermonecrotic toxin (DNT), and tracheal cytotoxin (TCT)—to safely prevent whooping cough while closely mimicking natural infection. This vaccine elicits robust Th1-biased cellular immunity alongside strong humoral responses. In a phase 2b human challenge study, intranasal BPZE1 vaccination prevented or markedly reduced infection following exposure to virulent B. pertussis, supporting its potential as a promising next-generation pertussis vaccine. Given its favorable safety profile, large-scale phase 3 clinical trials are warranted to confirm these findings and further assess its public health impact.
#pertussis
#pertussisvaccines
#mucosalvaccines
#nasalvaccines
https://lnkd.in/gukzy-aE

|
Scooped by
Gilbert C FAURE
December 24, 2025 8:10 AM
|
A mother's touch: microbial guardians of early immune imprinting
Trends in Immunology, Month 2025, Vol. xx, No. xx https://lnkd.in/gJuDgB4B
Group 3 Innate Lymphoid Cells (ILC3s) are a firmly established and universally accepted concept in immunology. They are considered the "innate counterparts" to T helper 17 (Th17) cells, as both share the master transcription factor, Retinoid-Related Orphan Receptor gamma t, and produce the cytokines IL-17 and IL-22.
This review:
· The Biological Paradigm: Infants are born with a pre-established "immune memory" despite developing in a relatively sterile environment. This in utero priming is driven by the maternal immune system and microbiota through a multi-modal exchange involving maternal microchimeric cells, IgG transfer, and microbiota-derived metabolites/antigens.
· Mechanisms of Action: Recent murine and human data demonstrate that maternal microbial products cross the placental barrier to actively fine-tune the fetal immune system. This results in antigen-specific immunological memory and epigenetic imprinting, effectively "programming" fetal immune cells before birth.
· Clinical Significance: This delicate priming process is highly susceptible to external stressors. Maternal dysbiosis, driven by diet, antibiotics, or infection, can disrupt these pathways, leading to aberrant immune development. Such prenatal perturbations are increasingly linked to the pathogenesis of autoimmune and immune-mediated disorders later in life.
Figure: Maternal factors that shape fetal immune priming.
During gestation, microbial and dietary antigens are trafficked across the placenta, partially via neonatal Fc receptor (FcRn)-mediated transport of immune complexes. These antigens are taken up by fetal dendritic cells (DCs) for priming of T cells, which are predisposed to differentiate into regulatory T cells (Tregs), thus establishing a level of immune tolerance toward commensals and dietary antigens before birth. Microbiota-associated metabolites, such as short-chain fatty acids (SCFAs) and tryptophan (Trp) derivatives, also cross the placenta and contribute to Treg priming, while maternal microchimeric cells take up residence in fetal tissues and promote tolerance of maternal antigens. Maternal interleukin (IL)-10 further promotes a tolerogenic immune milieu in the fetus, while, in situations of maternal immune activation (MIA), cytokines, such as IL-6 and IL-17, may skew newly activated T cells toward a more inflammatory profile, and alter the development of the intestinal epithelium and central nervous system. Dotted lines indicate pathways that are speculative.

|
Scooped by
Gilbert C FAURE
December 20, 2025 2:17 AM
|
|

|
Scooped by
Gilbert C FAURE
February 21, 4:02 AM
|

|
Scooped by
Gilbert C FAURE
February 17, 12:55 PM
|
Harnessing Mucosal Immunity for Protective Vaccines -
A thorough review on mucosal immunity, the type of responses elicited, the unique anatomical and immunological features of the mucosal surfaces of the body, and the challenges associated with the generation of protective immunity via mucosal vaccines.
https://sco.lt/8hqDuy
#vaccines #influenza #Covid19 #RSV #HMPV #HPIV #health #globalhealth #publichealth #medicine #biotechnology #medicine #pharmaceuticals #FDA #CDC #WHO #ECDC

|
Scooped by
Gilbert C FAURE
February 15, 1:39 PM
|
What does it mean if we find measles virus in a SEWER? I’m a few weeks into my new role as Vermont’s State Epidemiologist for Infectious Diseases and last week we detected measles virus in wastewater in the state, even though our team hadn’t found any cases of measles since last spring.
Measles virus isn’t usually in wastewater, so finding it there means that someone had measles in the area. They might have been a resident or someone traveling through (and who wouldn’t want to travel through Vermont??).
Fortunately, our epidemiology team has many “streams” of data (get it?). So, a few days later, we were able to identify a case of measles in a person living in the same area. Our epidemiologists connected with the patient to help prevent further spread.
We now know that wastewater is working for us as an early detection system. With rising measles throughout the United States, we will be keeping a close eye on it.
https://lnkd.in/gRQguCwB

|
Scooped by
Gilbert C FAURE
February 4, 6:43 AM
|
Each year, vast clouds of dust journey from the Sahara to Europe. But they don't travel alone. They carry a hidden cargo of millions of microbes.
Now, a team from the University of Lisbon, powered by MGI's sequencing tools, is investigating how this invisible migration is reshaping Portuguese agriculture. Their discovery during Storm Célia—a bacterial genus with potential as a powerful bio-fertilizer—turns an environmental phenomenon into a beacon of biotechnological hope.

|
Scooped by
Gilbert C FAURE
February 1, 12:53 PM
|
Prior seasonal influenza virus immunity did not impair antibody responses or protection conferred by the intranasal H5N1 vaccine.

|
Scooped by
Gilbert C FAURE
January 14, 7:48 AM
|
GUT MICROBIOTA IN EARLY CHILDHOOD DEPENDS ON THE MICROBIOTA OF BREAST'S MILK
The establishment of the gut microbiome in early life is critical for healthy infant development.
Mother's milk is crucial for shaping the infant gut microbiome by delivering beneficial bacteria, prebiotics, antibodies, and immune cells, fostering the growth of helpful microbes like Bifidobacterium and reducing pathogens, which is vital for immune development, nutrient absorption, and protection against chronic diseases.
This maternal transfer, via a gut-milk-infant pathway, helps establish a stable, healthy gut ecosystem that supports long-term health.
In an Open Access paper in Nature Communications, the results of an important study on the relationship between intestinal microbiota and breast milk in early childhood.
In this study, the authors quantified the similarity between the maternal milk and the infant gut microbiomes.
They used 507 metagenomic samples collected from 195 mother-infant pairs at one, three, and six months postpartum.
Microbial taxonomic overlap between milk and the infant gut was driven by Bifidobacterium longum, and infant microbiomes dominated by B. longum showed greater temporal stability than those dominated by other species.
They also identified numerous instances of strain sharing between milk and the infant gut, involving both commensal (e.g. B. longum) and pathobiont species (e.g. K. pneumoniae).
Shared strains also included typically oral species such as S. salivarius and V. parvula, suggesting possible transmission from the infant’s oral cavity to the mother’s milk.
At one month, the infant gut microbiome was enriched in biosynthetic pathways, suggesting that early colonisers might be more metabolically independent than those present at six months.
Lastly, they observed significant overlap in antimicrobial resistance gene carriage within mother-infant pairs.
Together, these results suggest that the human milk microbiome has an important role in the assembly, composition, and stability of the infant gut microbiome.
Ferretti, P., Allert, M., Johnson, K.E. et al. Nat Commun 16, 11536 (2025). https://lnkd.in/eD92fRkM

|
Scooped by
Gilbert C FAURE
January 12, 8:57 AM
|
Deux études parues dans « Nature Cancer » décrivent comment des niveaux élevés de bactéries infiltrant les tumeurs affaiblissent la réponse immunitaire, favorisant une résistance à l’immunothérapie dans les cancers de la tête et du cou.

|
Scooped by
Gilbert C FAURE
January 7, 4:45 AM
|
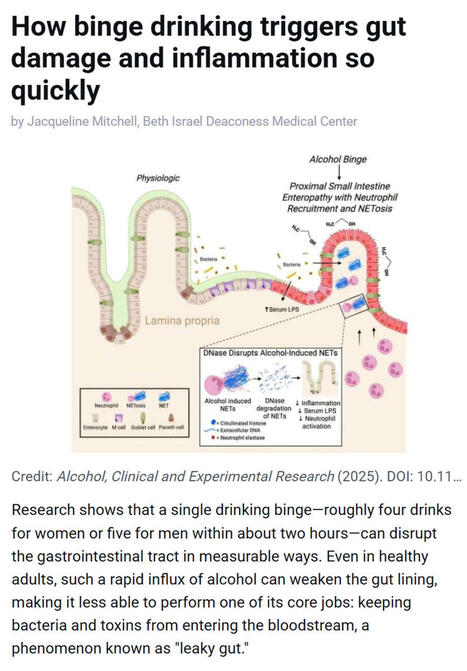
Research shows that even a single episode of binge drinking - about four drinks for women or five for men within two hours - can weaken the gut barrier, allowing #bacteria and #toxins to enter the bloodstream and trigger #inflammation, a process often referred to as “leaky gut.”
▫️ Investigators at Beth Israel Deaconess Medical Center (BIDMC), in work published in Alcohol: Clinical and Experimental Research, found that short bursts of high-dose #alcohol recruit immune cells called #neutrophils to the upper small intestine, where they release damaging structures known as NETs that disrupt the gut lining.
▫️ NETs stands for Neutrophil Extracellular Traps. They are web-like structures made of #DNA, histones, and antimicrobial proteins that are released by neutrophils to trap and kill #microbes. While NETs are part of the body’s innate immune defense, they can also damage surrounding tissues when produced excessively or inappropriately - such as after binge alcohol exposure - by disrupting barriers like the gut lining, promoting inflammation, and allowing bacteria or toxins to leak into the bloodstream.
▫️ The study, led by Scott Minchenberg, MD, PhD, a clinical fellow in #gastroenterology and #hepatology at BIDMC, showed that breaking down these NETs with an enzyme reduced gut damage and bacterial leakage.
▫️ As noted by senior author Gyongyi Szabo MD, PhD, Chief Academic Officer at BIDMC and Beth Israel Lahey Health, these findings highlight an early inflammatory pathway linking binge drinking to gut and liver injury.
🗃️ See comments section for reference.

|
Scooped by
Gilbert C FAURE
January 4, 3:51 AM
|
Mucosal glycans: key drivers of the development of inflammatory bowel disease and a potential new therapeutic target - Nature Reviews Gastroenterology & Hepatology

|
Scooped by
Gilbert C FAURE
January 1, 4:29 AM
|
Une découverte inattendue sur notre immunité ?
Des chercheurs japonais révèlent un rôle peu exploré de la salive.
📌 Ce qu’il faut savoir
Une équipe de l’Université de Tokyo a analysé la salive de 476 volontaires.
Leurs travaux, publiés dans Nature Communications, identifient des fragments génétiques jusqu’ici peu décrits, portés par certaines bactéries de la bouche.
➡️ Ces fragments, appelés Inocles, sont présents chez près de 3 personnes sur 4.
Il s’agit de petits morceaux d’ADN supplémentaires, distincts de l’ADN principal des bactéries.
Ils ne sont pas indispensables à leur survie, mais semblent leur conférer des capacités d’adaptation accrues, notamment pour faire face aux contraintes constantes de l’environnement buccal (alimentation, acidité, hygiène…).
👉 Pourquoi est-ce important ?
Parce que la bouche n’est pas qu’un simple point de passage. C’est un écosystème biologique dense et actif, où :
-un microbiote complexe cohabite en permanence
-certaines bactéries interagissent avec notre organisme
-ces interactions pourraient être associées à des variations de la réponse immunitaire
Ce que les chercheurs ont observé chez les personnes porteuses d’Inocles :
-une activité immunitaire différente
-notamment au niveau de cellules clés de l’immunité adaptative
Autrement dit, ce qui se passe dans la bouche pourrait influencer la manière dont notre système immunitaire se régule.
Quels liens ont-ils fait avec le cancer ?
Les chercheurs ont également observé que certaines personnes atteintes de certains cancers présentaient moins d’Inocles.
Il ne s’agit ni d’un lien causal, ni d’un traitement, mais d’une piste de recherche encore très précoce.
⚠️ À ce stade, ces résultats sont observationnels. Ils ouvrent de nouvelles questions, mais nécessitent encore de nombreuses études pour être confirmés. | 37 comments on LinkedIn

|
Scooped by
Gilbert C FAURE
December 27, 2025 10:43 AM
|
Head and neck #cancer is the seventh most common cancer worldwide.
This JAMA Review summarizes the epidemiology, clinical presentation, diagnosis, and treatment of head and neck squamous cell carcinomas (#HNSCC) of the upper aerodigestive tract.
https://ja.ma/4q6rxjn

|
Scooped by
Gilbert C FAURE
December 21, 2025 4:08 AM
|
Just as a curiosity.
"Chris Buck stands barefoot in his kitchen holding a glass bottle of unfiltered Lithuanian farmhouse ale. He swirls the bottle gently to stir up a fingerbreadth blanket of yeast and pours the turbulent beer into a glass mug.
Buck raises the mug and sips. “Cloudy beer. Delightful!”
He has just consumed what may be the world’s first vaccine delivered in a beer. It could be the first small sip toward making vaccines more palatable and accessible to people around the world. Or it could fuel concerns about the safety and effectiveness of vaccines. Or the idea may go nowhere. No matter the outcome, the story of Buck’s unconventional approach illustrates the legal, ethical, moral, scientific and social challenges involved in developing potentially life-saving vaccines."
https://lnkd.in/gdPZRTsV

|
Scooped by
Gilbert C FAURE
December 18, 2025 4:23 AM
|
Now published open access. ‘Since the early 1950s, national statisticians have regarded unpaid work as non-economic, excluding it from GDP. Feminist scholars argue this exclusion reflects a gender-biased view of progress that renders women’s non-market productivity invisible. As what gets measured drives policy priorities and resource allocation, breastfeeding highlights the need to account for women’s unpaid care work in economic statistics. This paper advances the Beyond GDP agenda by demonstrating how market-derived prices can improve the measurement and recognition of women’s lactation labour.’
|






 Your new post is loading...
Your new post is loading...