Neuromodulation technologies are crucial for investigating neuronal connectivity and brain function. Magnetic neuromodulation offers wireless and remote deep brain stimulations that are lacking in optogenetic- and wired-electrode-based tools. However, due to the limited understanding of working principles and poorly designed magnetic operating systems, earlier magnetic approaches have yet to be utilized. Furthermore, despite its importance in neuroscience research, cell-type-specific magnetic neuromodulation has remained elusive. Here we present a nanomaterials-based magnetogenetic toolbox, in conjunction with Cre-loxP technology, to selectively activate genetically encoded Piezo1 ion channels in targeted neuronal populations via torque generated by the nanomagnetic actuators in vitro and in vivo. We demonstrate this cell-type-targeting magnetic approach for remote and spatiotemporal precise control of deep brain neural activity in multiple behavioural models, such as bidirectional feeding control, long-term neuromodulation for weight control in obese mice and wireless modulation of social behaviours in multiple mice in the same physical space. Our study demonstrates the potential of cell-type-specific magnetogenetics as an effective and reliable research tool for life sciences, especially in wireless, long-term and freely behaving animals. Minimally invasive cellular-level target-specific neuromodulation is needed to decipher brain function and neural circuitry. Here nano-magnetogenetics using magnetic force actuating nanoparticles has been reported, enabling wireless and remote stimulation of targeted deep brain neurons in freely behaving animals.





 Your new post is loading...
Your new post is loading...





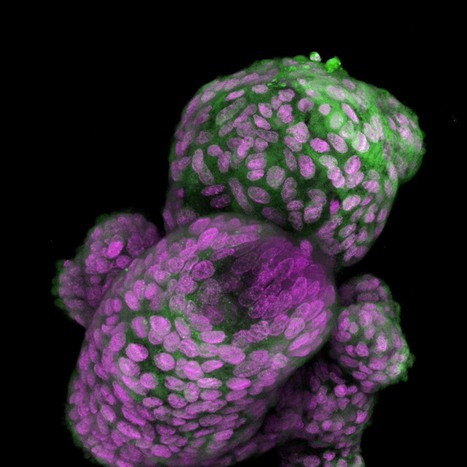

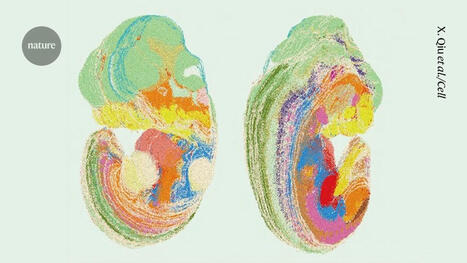









The researchers found ways to recreate a simplified version of gastrulation in a dish by starting with a layer of induced pluripotent stem (iPS) cells, meaning they can differentiate to become any cell type in the body. Next, the scientists added a protein called BMP4, a key signaling molecule in gastrulation, which causes the cells in the box to begin forming the three layers of cells present in the embryo. All cells appear to receive the same BMP4 signal, however, some transform into one cell type while others become different cell types. When creating a gastrulation model, researchers observed that iPS cells contain proteins that are the building blocks of tight junctions. They also noted that tight junctions do not always assemble, and that tight junctions between adjacent cells appear to render cells impervious to BMP4 signals. To confirm the importance of tight junctions in gastrulation, the researchers used CRISPR genome-editing technology to suppress the production of TJP1, a protein crucial for tight junction formation in iPS cells. When they applied BMP4 to cells lacking the TJP1 protein, every cell was activated, not just the peripubic cells. This discovery forms the basis of a new method for efficiently producing these unique cells.