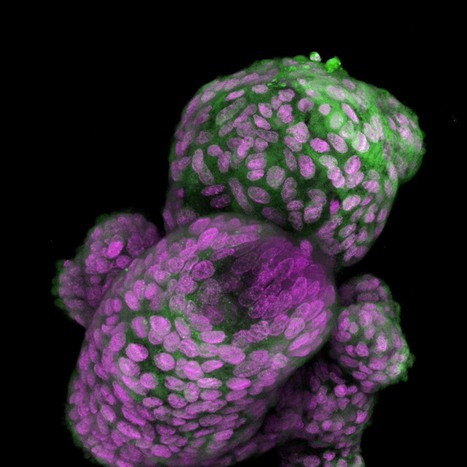
Neuromodulation technologies are crucial for investigating neuronal connectivity and brain function. Magnetic neuromodulation offers wireless and remote deep brain stimulations that are lacking in optogenetic- and wired-electrode-based tools. However, due to the limited understanding of working principles and poorly designed magnetic operating systems, earlier magnetic approaches have yet to be utilized. Furthermore, despite its importance in neuroscience research, cell-type-specific magnetic neuromodulation has remained elusive. Here we present a nanomaterials-based magnetogenetic toolbox, in conjunction with Cre-loxP technology, to selectively activate genetically encoded Piezo1 ion channels in targeted neuronal populations via torque generated by the nanomagnetic actuators in vitro and in vivo. We demonstrate this cell-type-targeting magnetic approach for remote and spatiotemporal precise control of deep brain neural activity in multiple behavioural models, such as bidirectional feeding control, long-term neuromodulation for weight control in obese mice and wireless modulation of social behaviours in multiple mice in the same physical space. Our study demonstrates the potential of cell-type-specific magnetogenetics as an effective and reliable research tool for life sciences, especially in wireless, long-term and freely behaving animals. Minimally invasive cellular-level target-specific neuromodulation is needed to decipher brain function and neural circuitry. Here nano-magnetogenetics using magnetic force actuating nanoparticles has been reported, enabling wireless and remote stimulation of targeted deep brain neurons in freely behaving animals.





 Your new post is loading...
Your new post is loading...




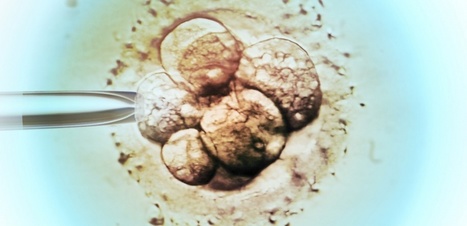


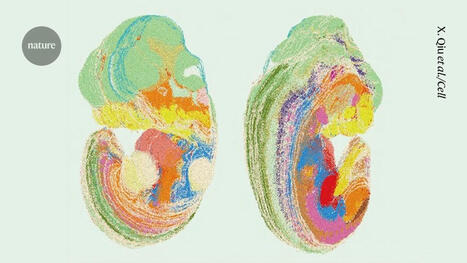


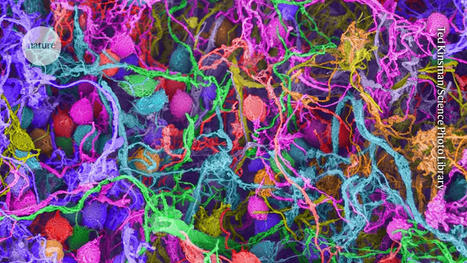

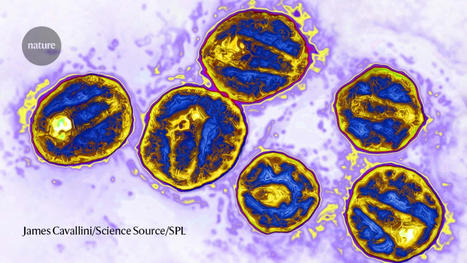






Researchers have created a laboratory-developed, three-dimensional organoid model derived from human tissue and designed to understand the early stages of cancer in the gastroesophageal junction (GEJ), the point where the food pipe of the digestive system meets the stomach. Using CRISPR-Cas9, the researchers also eliminated two key tumor suppressor genes, TP53 and CDKN2A,c in the organoids. The dual inactivation of these genes made the cells more cancerous, with faster growth and microscopic features closer to malignancy. These altered organoids also formed tumors in immunodeficient mice. The team further found abnormalities in lipids that store energy but also perform a variety of other functions, and identified platelet activating factor as a key lipid upregulated in GEJ organoids. The researchers used WEB2086, which stopped the growth of implanted GEJ organoid tumors. WEB2086, a Food and Drug Administration-approved compound used to treat platelet diseases, inhibits platelet activating factor receptors in GEJ.