Neuromodulation technologies are crucial for investigating neuronal connectivity and brain function. Magnetic neuromodulation offers wireless and remote deep brain stimulations that are lacking in optogenetic- and wired-electrode-based tools. However, due to the limited understanding of working principles and poorly designed magnetic operating systems, earlier magnetic approaches have yet to be utilized. Furthermore, despite its importance in neuroscience research, cell-type-specific magnetic neuromodulation has remained elusive. Here we present a nanomaterials-based magnetogenetic toolbox, in conjunction with Cre-loxP technology, to selectively activate genetically encoded Piezo1 ion channels in targeted neuronal populations via torque generated by the nanomagnetic actuators in vitro and in vivo. We demonstrate this cell-type-targeting magnetic approach for remote and spatiotemporal precise control of deep brain neural activity in multiple behavioural models, such as bidirectional feeding control, long-term neuromodulation for weight control in obese mice and wireless modulation of social behaviours in multiple mice in the same physical space. Our study demonstrates the potential of cell-type-specific magnetogenetics as an effective and reliable research tool for life sciences, especially in wireless, long-term and freely behaving animals. Minimally invasive cellular-level target-specific neuromodulation is needed to decipher brain function and neural circuitry. Here nano-magnetogenetics using magnetic force actuating nanoparticles has been reported, enabling wireless and remote stimulation of targeted deep brain neurons in freely behaving animals.





 Your new post is loading...
Your new post is loading...




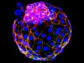

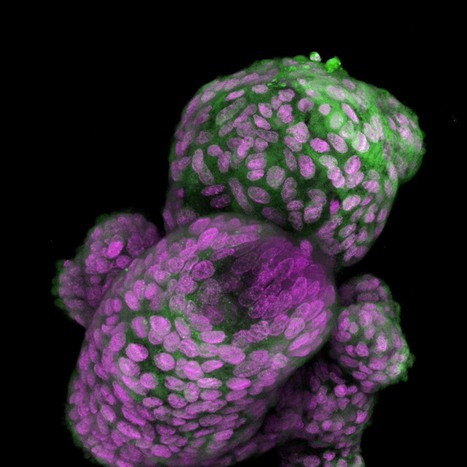
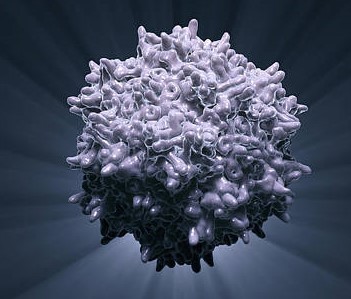

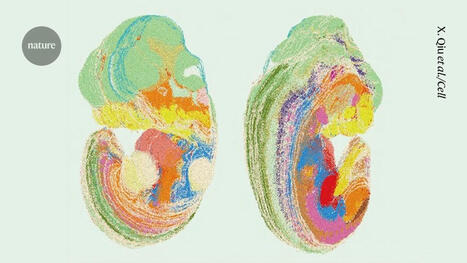


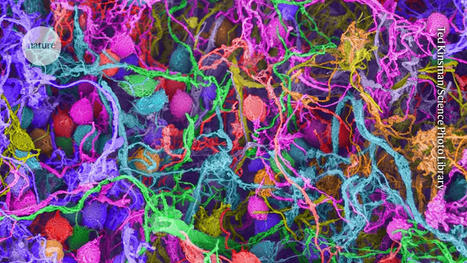

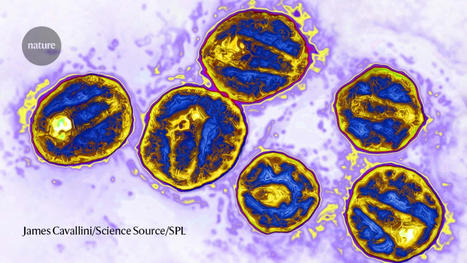
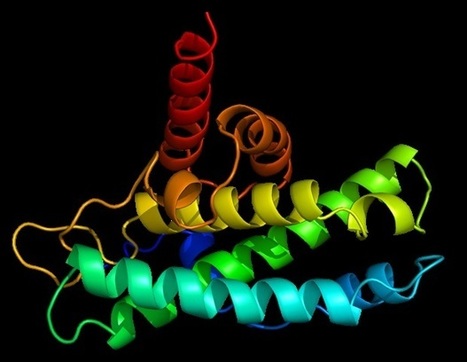






Scientists set out to prove experimentally what they had first deduced from computational studies: that the apple plasminogen-nematode domain, or PAN, is linked to cell proliferation that drives tumor growth in humans and defense signaling during plant-microbe interactions in bioenergy crops. The association was first made when researchers explored the genomes of crops like poplar and willow. In the latest study, the research team identified four core amino acids called cysteine residues in the HGF protein essential for PAN domain function and studied their behavior in human cancer cell lines. They found that mutation of one of these amino acids, achieved using CRISPR-9, disabled the signaling pathway known as HGF-c-MET that is abnormally high in cancer cells, forcing them to multiply and spread rapidly. The team's findings, published in Nature Communications Biology, open a new avenue for the development of selective drug therapies to fight a variety of cancers such as those that start in the breast and stomach.