Neuromodulation technologies are crucial for investigating neuronal connectivity and brain function. Magnetic neuromodulation offers wireless and remote deep brain stimulations that are lacking in optogenetic- and wired-electrode-based tools. However, due to the limited understanding of working principles and poorly designed magnetic operating systems, earlier magnetic approaches have yet to be utilized. Furthermore, despite its importance in neuroscience research, cell-type-specific magnetic neuromodulation has remained elusive. Here we present a nanomaterials-based magnetogenetic toolbox, in conjunction with Cre-loxP technology, to selectively activate genetically encoded Piezo1 ion channels in targeted neuronal populations via torque generated by the nanomagnetic actuators in vitro and in vivo. We demonstrate this cell-type-targeting magnetic approach for remote and spatiotemporal precise control of deep brain neural activity in multiple behavioural models, such as bidirectional feeding control, long-term neuromodulation for weight control in obese mice and wireless modulation of social behaviours in multiple mice in the same physical space. Our study demonstrates the potential of cell-type-specific magnetogenetics as an effective and reliable research tool for life sciences, especially in wireless, long-term and freely behaving animals. Minimally invasive cellular-level target-specific neuromodulation is needed to decipher brain function and neural circuitry. Here nano-magnetogenetics using magnetic force actuating nanoparticles has been reported, enabling wireless and remote stimulation of targeted deep brain neurons in freely behaving animals.





 Your new post is loading...
Your new post is loading...




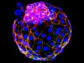

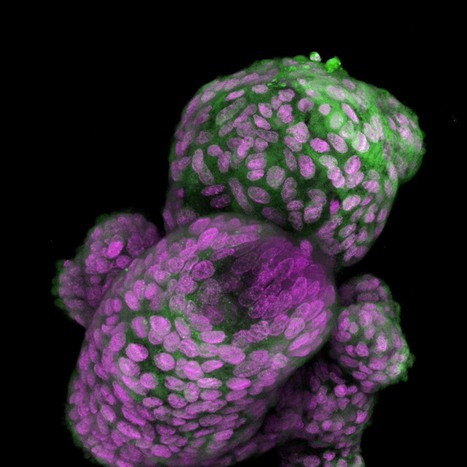

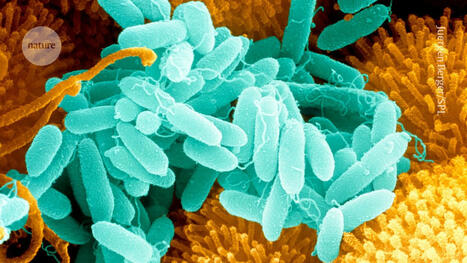








Within the defense mechanism of bacteria, the so-called type III variants of the CRISPR gene produce small signal molecules. With the help of these small molecules, bacteria trigger a complex contingency plan that ensures that a virus can be fought optimally and on a broad front. Researchers have studied how this works and have discovered that the small signal molecules bind, among others, to a protein called CalpL, which thus becomes an active "protease". Proteases are enzymes that cleave proteins and thus function as protein scissors. Proteases are also used in the human immune system to transmit information at high speed. Finally, the researchers also found the target of their newly discovered protein scissors. It cuts a small protein molecule called CalpT, which acts as a safety lock for CalpS, a third protein molecule. CalpS is a very well-guarded protein that is released by the whole mechanism. It will drive the transcription machinery to specific genes, switching the bacteria's metabolism to defense. With the discovery of this complicated signaling cascade, the researchers have now uncovered a whole new aspect of CRISPR systems. The study was published in the renowned scientific journal "Nature".