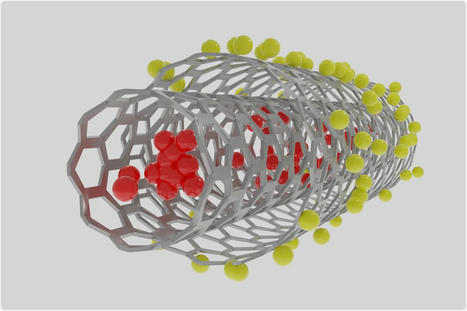
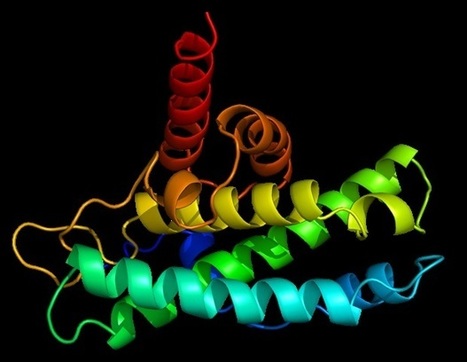
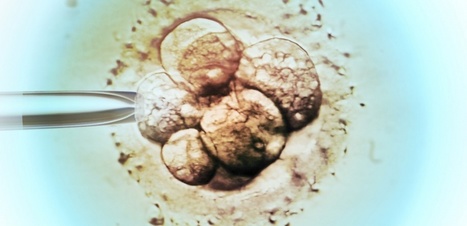
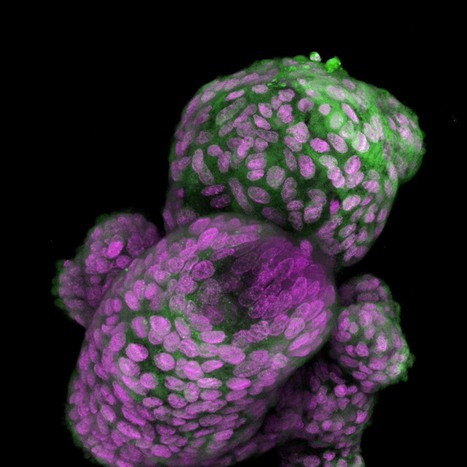
Neuromodulation technologies are crucial for investigating neuronal connectivity and brain function. Magnetic neuromodulation offers wireless and remote deep brain stimulations that are lacking in optogenetic- and wired-electrode-based tools. However, due to the limited understanding of working principles and poorly designed magnetic operating systems, earlier magnetic approaches have yet to be utilized. Furthermore, despite its importance in neuroscience research, cell-type-specific magnetic neuromodulation has remained elusive. Here we present a nanomaterials-based magnetogenetic toolbox, in conjunction with Cre-loxP technology, to selectively activate genetically encoded Piezo1 ion channels in targeted neuronal populations via torque generated by the nanomagnetic actuators in vitro and in vivo. We demonstrate this cell-type-targeting magnetic approach for remote and spatiotemporal precise control of deep brain neural activity in multiple behavioural models, such as bidirectional feeding control, long-term neuromodulation for weight control in obese mice and wireless modulation of social behaviours in multiple mice in the same physical space. Our study demonstrates the potential of cell-type-specific magnetogenetics as an effective and reliable research tool for life sciences, especially in wireless, long-term and freely behaving animals. Minimally invasive cellular-level target-specific neuromodulation is needed to decipher brain function and neural circuitry. Here nano-magnetogenetics using magnetic force actuating nanoparticles has been reported, enabling wireless and remote stimulation of targeted deep brain neurons in freely behaving animals.




 Your new post is loading...
Your new post is loading...









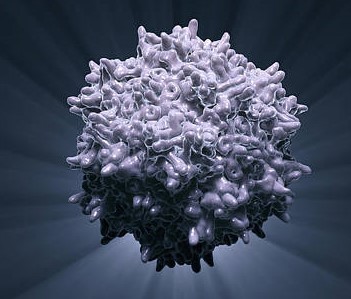






Therapeutic nanosystems and diagnostic systems are increasingly being applied to various areas of medicine, acting as sensors, delivery vehicles, immunostimulants, radiation sensitizers and viral inhibitors. In an article recently published in the journal Pharmaceutics, the application of nanomaterials in the diagnosis, prevention and treatment of viral diseases is reviewed in detail, highlighting areas of significant progress or stagnation over the past decades. Drug delivery is the primary focus of nanomaterials in disease treatment, generally offering improved pharmacokinetics, drug retention time, and "drug-like" of the administered compound. The physical and chemical properties of the nanoparticle strongly influence the biodistribution of the nanomedicine in the body and the ability and propensity of the particle to enter the target cells. In addition to being used as a delivery vehicle, the nanoparticles themselves can be used as viral therapy, acting to block the viral replication cycle or cell entry. Particles constructed from copper, silver and gold are also capable of generating reactive oxygen species. In addition to being directly damaging to viral genetic material, it can induce apoptosis in infected cells, thus preventing viral propagation.