B-cell receptor (BCR) complexes are expressed on the surface of a B-cell and are critical in antigen recognition and modulating the adaptive immune response. Even though the relevance of antibodies has been known for almost a hundred years, the antigen-dependent activation mechanism of B-cells has remained elusive. Several models have been proposed for BCR activation, including cross-linking, conformation-induced oligomerization, and dissociation activation models. Recently, the first cryo-EM structures of the human B-cell antigen receptor of the IgM and IgG isotypes have been published that validates the asymmetric organization of the BCR complex. Here, we carry out extensive molecular dynamics simulations to probe the conformational changes upon antigen binding and the influence of the membrane lipids. We identify two critical dynamical events that could be associated with antigen-dependent activation of BCR. First, antigen binding causes increased flexibility in regions distal to the antigen binding site. Second, antigen binding alters the rearrangement of IgM transmembrane helices, including the relative interaction of Igα/Igβ that mediates intracellular signaling. Furthermore, these transmembrane rearrangements lead to changes in localized lipid composition. Our work indirectly supports the conformational-change induced models of BCR activation and contributes to the understanding of the antigen-dependent activation mechanism of BCRs. Extensive multiscale modeling of B-cell receptor in the presence and absence of a model antigen in a complex membrane environment revealed the dynamical events throughout the receptor and surrounding membrane upon antigen binding to the receptor.
Follow, research and publish the best content
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account
Already have an account: Login
Teaching and Learning Immunology. Information you never would have searched for!
Curated by
Gilbert C FAURE
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
|
|



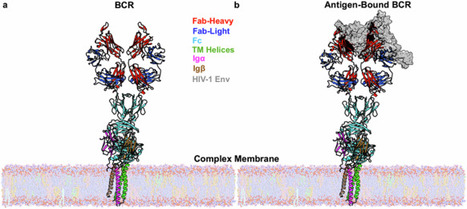


















Antibodies despite being a cornerstone of modern medicine and driving a multibillion-dollar industry, we still don’t fully understand the fundamental mechanisms behind the production of these fascinating molecules.
A recent study published in Communications Biology combines Cryo-EM experiments and Molecular Dynamics simulations to propose a new, comprehensive theory of BCR (pre-antibody) dynamics. The study suggests that the interaction between pre-IgM and Ig alpha/beta is asymmetric, challenging previous assumptions.
Additionally, it highlights the role of oligomerization surfaces that appear and disappear in BCRs—if confirmed, this could have significant implications for the development of therapeutic antibodies and our understanding of immune health and disease.
This exciting work needs further experimental validation, particularly in autoimmune diseases. After more than 120 years of immunology research, this field continues to surprise us!