Your new post is loading...
 Your new post is loading...

|
Scooped by
HAS-veille
October 19, 2022 8:25 AM
|
Males with X-linked adrenoleukodystrophy (ALD) are at high risk for developing adrenal insufficiency and/or progressive leukodystrophy (cerebral ALD) at an early age. Pathogenic variants in ABCD

|
Scooped by
HAS-veille
October 14, 2022 8:11 AM
|
In the Netherlands, abnormal New-Born Screening (NBS) results are communicated to parents by the general practitioner (GP). Good communication and consequential trust in professionals is of the utmost importance in the treatment of phenylketonuria (PKU). The aim of this study was to assess parental satisfaction regarding the communication of an abnormal NBS result for PKU in the Netherlands. An email containing the link to a web-based questionnaire was sent by the Dutch PKU Association to their members. Responses to open questions were categorized, data of both open and closed questions were analysed with descriptive statistics and the Chi-Square test using SPSS. Out of 113 parents of a child with PKU (born between 1979 and 2020), 68 stated they were overall unsatisfied with the first communication of the NBS result. Seventy-five parents indicated that wrong or no information about PKU was given. A significant decrease was found in the number of parents being contact by their own GP over the course of 40 years (p < 0.05). More than half of all parents were overall unsatisfied with the first communication of the abnormal NBS result for PKU. Further research on how to optimize communication of an abnormal NBS results is necessary.

|
Scooped by
HAS-veille
October 11, 2022 9:53 AM
|
It has recently been announced that the Secretary of the U.S. Department of Health and Human Services has approved the recommendation by the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) to add mucopolysaccharidosis type II (MPS-II, Hunter Syndrome) to the recommended uniform screening panel (RUSP) in the United States [...]

|
Scooped by
HAS-veille
October 3, 2022 4:57 AM
|

|
Scooped by
HAS-veille
September 27, 2022 7:27 AM
|
Our objective was to develop and test a new approach to obtaining parental policy guidance about disclosure of incidental findings of newborn screening for cystic fibrosis (CF), including heterozygote carrier status and the conditions known as CFTR-related metabolic syndrome (CRMS) and/or cystic fibrosis screen positive inconclusive diagnosis, CFSPID. The participants were parents of infants up to 6 months old recruited from maternity hospitals/clinics, parent education classes and stores selling baby products. Data were collected using an anonymous, one-time Internet-based survey. The survey introduced two scenarios using novel, animated videos. Parents were asked to rank three potential disclosure policies—Fully Informed, Parents Decide, and Withholding Information. Regarding disclosure of information about Mild X (analogous to CRMS/CFSPID), 57% of respondents ranked Parents Decide as their top choice, while another 41% ranked the Fully Informed policy first. Similarly, when considering disclosure of information about Disease X (CF) carrier status, 50% and 43% gave top rankings to the Fully Informed and Parents Decide policies, respectively. Less than 8% ranked the Withholding Information policy first in either scenario. Data from value comparisons suggested that parents believed knowing everything was very important even if they became distressed. Likewise, parents preferred autonomy even if they became distressed. However, when there might not be enough time to learn everything, parents showed a slight preference for deferring decision-making. Because most parents strongly preferred the policies of full disclosure or making the decision, rather than the withholding option for NBS results, these results can inform disclosure policies in NBS programs, especially as next-generation sequencing increases incidental findings.

|
Scooped by
HAS-veille
September 26, 2022 6:46 AM
|
Newborn screening was established over 50 years ago to identify cases of disorders that were serious, urgent, and treatable, mirroring the criteria of Wilson and Jungner. In the last decade, conditions have been added to newborn screening that do not strictly meet these criteria, and genomic newborn screening is beginning to be discussed. Some of these new and proposed additions to newborn screening entail serious public health ethical issues that need to be explored.

|
Scooped by
HAS-veille
September 23, 2022 2:01 AM
|
The National MPS Society, Inc., founded in 1974, is a rare disease advocacy non-profit with a tripartite mission addressing the needs of the mucopolysaccharidosis and mucolipidosis communitie

|
Scooped by
HAS-veille
September 20, 2022 3:57 AM
|
Background We present the results of our experience in the diagnosis and follow up of the positive cases for propionic, methylmalonic acidemias and cobalamin deficiencies (PA/MMA/MMAHC) since the Expanded Newborn Screening was implemented in Madrid Region. Methods Dried blood samples were collected 48 h after birth. Amino acids and acylcarnitines were quantitated by MS/MS. Newborns with alterations were referred to the clinical centers for follow-up. Biochemical and molecular genetic studies for confirmation of a disease were performed. Results In the period 2011–2020, 588,793 children were screened, being 953 of them were referred to clinical units for abnormal result (192 for elevated C3 levels). Among them, 88 were false positive cases, 85 maternal vitamin B12 deficiencies and 19 were confirmed to suffer an IEM (8 PA, 4 MMA, 7 MMAHC). Ten out 19 cases displayed symptoms before the NBS results (6 PA, 1 MMA, 3 MMAHC). C3, C16:1OH+C17 levels and C3/C2 and C3/Met ratios were higher in newborns with PA/MMA/MMAHC. Cases diagnosed with B12 deficiency had mean B12 levels of 187.6 ± 76.9 pg/mL and their mothers 213.7 ± 95.0; 5% of the mothers were vegetarian or had poor eating while 15% were diagnosed of pernicious anemia. Newborns and their mothers received treatment with B12 with different posology, normalizing their levels and the secondary alterations disappeared. Conclusions Elevated C3 are a frequent cause for abnormal result in newborn screening with a high rate of false positive cases. Presymptomatic diagnosis of most of PA and some MMA/MMAHC is difficult. Vitamin B12 deficiency secondary to maternal deprivation is frequent with an heterogenous clinical and biochemical spectrum.

|
Scooped by
HAS-veille
September 15, 2022 3:54 AM
|
Newborn screening (NBS) is a successful public health initiative that effectively identifies pre-symptomatic neonates so that treatment can be initiated before the onset of irreversible morbidit

|
Scooped by
HAS-veille
August 30, 2022 2:21 AM
|
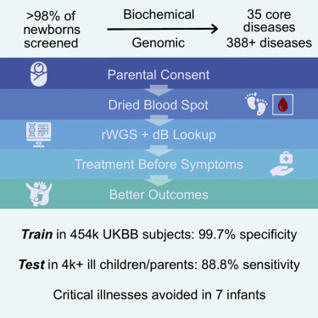
Newborn screening (NBS) dramatically improves outcomes in severe childhood disorders by treatment before symptom onset. In many genetic diseases, howe…

|
Scooped by
HAS-veille
August 12, 2022 2:17 AM
|
Spinal muscular atrophy (SMA) is a rare genetic disorder that causes progressive muscle weakness and paralysis. In its most common and severe form, th…

|
Scooped by
HAS-veille
August 9, 2022 7:03 AM
|
Newborn screening (NBS) for inborn errors of metabolism is one of the most advanced tools for secondary prevention in medicine, as it allows early diagnosis and prompt treatment initiation. The expanded newborn screening was introduced in Italy between 2016 and 2017 (Law 167/2016; DM 13 October 2016; DPCM 12-1-2017). A total of 1,586,578 infants born in Italy were screened between January 2017 and December 2020. For this survey, we collected data from 15 Italian screening laboratories, focusing on the metabolic disorders identified by tandem mass spectrometry (MS/MS) based analysis between January 2019 and December 2020. Aminoacidemias were the most common inborn errors in Italy, and an equal percentage was observed in detecting organic acidemias and mitochondrial fatty acids beta-oxidation defects. Second-tier tests are widely used in most laboratories to reduce false positives. For example, second-tier tests for methylmalonic acid and homocysteine considerably improved the screening of CblC without increasing unnecessary recalls. Finally, the newborn screening allowed us to identify conditions that are mainly secondary to a maternal deficiency. We describe the goals reached since the introduction of the screening in Italy by exchanging knowledge and experiences among the laboratories.

|
Scooped by
HAS-veille
August 9, 2022 3:40 AM
|
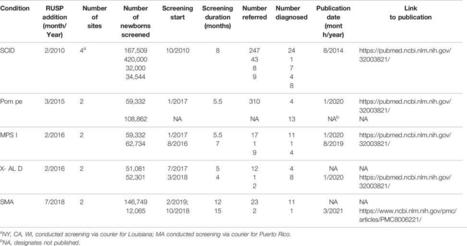
Each year, through population-based newborn screening (NBS), 1 in 294 newborns is identified with a condition leading to early treatment and, in some cases, life-saving interventions. Rapid advancements in genomic technologies to screen, diagnose, and treat newborns promise to significantly expand the number of diseases and individuals impacted by NBS. However, expansion of NBS occurs slowly in the United States (US) and almost always occurs condition by condition and state by state with the goal of screening for all conditions on a federally recommended uniform panel. The Newborn Screening Translational Research Network (NBSTRN) conducted the NBS Expansion Study to describe current practices, identify expansion challenges, outline areas for improvement in NBS, and suggest how models could be used to evaluate changes and improvements. The NBS Expansion Study included a workshop of experts, a survey of clinicians, an analysis of data from online repositories of state NBS programs, reports and publications of completed pilots, federal committee reports, and proceedings, and the development of models to address the study findings. This manuscript (Part One) reports on the design, execution, and results of the NBS Expansion Study. The Study found that the capacity to expand NBS is variable across the US and that nationwide adoption of a new condition averages 9.5 years. Four factors that delay and/or complicate NBS expansion were identified. A companion paper (Part Two
|

|
Scooped by
HAS-veille
October 14, 2022 8:12 AM
|
BackgroundNewborn screening (NBS) algorithms for cystic fibrosis (CF) vary in the USA and include different CFTR variants. CFTR variant distribution varies among racial and ethnic groups

|
Scooped by
HAS-veille
October 14, 2022 4:11 AM
|
Background Cystinuria is an inherited metabolic disease involving the defective transport of cystine and the dibasic amino acids in the renal proximal tubules that causes the formation of stones in the urinary system. In our regional child health program, cystinuria is included in newborn metabolic screening. Our objectives are the phenotypic characterization of our cystinuric pediatric cohort and to present our experience in neonatal cystinuria screening. Methods The study of clinical cases of pediatric patients diagnosed with cystinuria over a period of 32 years. All patients were studied at demographic, clinical, laboratory, radiological, and therapeutic levels. Results We diagnosed 86 pediatric patients with cystinuria; 36% of them had the homozygous biochemical phenotype. 95.3% of the patients were detected by neonatal metabolic screening. We performed urine biochemical analyses of parents with additional diagnoses of 63 adult patients. The mean follow-up time was 16.8 ± 8.5 years. 11.6% of patients developed one or more episodes of urinary tract infection during that period. Chronic kidney disease, proteinuria, and hypertension were uncommon (1.2%). 10.5% developed kidney stones at the mean age of presentation of 7.78 ± 7.6 years; 33% were recurrent. The risk of developing lithiasis was higher for homozygous biochemical-phenotype patients. Hypercalciuria was a significant risk factor in the development of lithiasis. Conclusions Our clinical data suggest that diagnosing cystinuria through neonatal screening could be a useful strategy for the detection of presymptomatic cases, in order to establish preventive measures, as well as for the detection of relatives at risk. Graphical abstract

|
Scooped by
HAS-veille
October 11, 2022 2:07 AM
|
This Special Issue provides a wide-ranging update from the front lines of newborn screening (NBS) research and is the result of conversations and collaborations facilitated by the Newborn Screenin

|
Scooped by
HAS-veille
September 27, 2022 9:46 AM
|
The purpose of this study is to provide the results of the newborn screening (NBS) program for Spinal Muscular Atrophy (SMA) in the state of Georgia to determine disease incidence, time to diagnosi

|
Scooped by
HAS-veille
September 27, 2022 7:15 AM
|
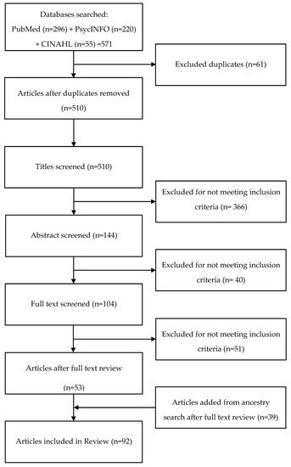
Genomic advances have contributed to a proliferation of newborn screening (NBS) programs. Psychosocial consequences of NBS have been identified as risks to these public health initiatives. Following PRISMA guidelines, this systematic review synthesizes findings from 92 evidence-based, peer-reviewed research reports published from 2000 through 2020 regarding psychosocial issues associated with NBS. Results describe parents’ knowledge of and attitudes towards NBS, reactions to and understanding of positive NBS results, experiences of communication with health providers, decisions about carrier testing, and future pregnancies. Findings also explain the impact of positive NBS results on parent–child relationships, child development, informing children about carrier status, family burden, quality of life, and disparities. In conclusion, psychosocial consequences of receiving unexpected neonatal screening results and unsolicited genetic information remain significant risks to expansion of NBS. Findings suggest that risks may be mitigated by improved parent NBS education, effective communication, individualized genetic counseling, and anticipatory developmental guidance. Clinicians need to take extra measures to ensure equitable service delivery to marginalized subpopulations. Future investigations should be more inclusive of culturally and socioeconomically diverse families and conducted in low-resource countries. Providing these countries with adequate resources to develop NBS programs is an essential step towards achieving international health equity.

|
Scooped by
HAS-veille
September 26, 2022 2:34 AM
|
Duchenne muscular dystrophy (DMD) is the most common pediatric-onset form of muscular dystrophy, occurring in 1 in 5,000 live male births. DMD is a multi-system disease resulting in muscle weaknes

|
Scooped by
HAS-veille
September 22, 2022 10:36 AM
|
Advancements in therapies for Duchenne muscular dystrophy (DMD) have made diagnosis within the newborn period a high priority. We undertook a consortia approach to advance DMD newborn screening in the United States. This manuscript describes the formation of the Duchenne Newborn Screening Consortium, the development of the pilot protocols, data collection tools including parent surveys, and findings from the first year of a two-year pilot. The DMD pilot design is population-based recruitment of infants born in New York State. Data tools were developed to document the analytical and clinical validity of DMD NBS, capture parental attitudes, and collect longitudinal health information for diagnosed newborns. Data visualizations were updated monthly to inform the consortium on enrollment. After 12 months, 15,754 newborns were screened for DMD by the New York State Newborn Screening (NYS NBS) Program. One hundred and forty screened infants had borderline screening results, and sixteen infants were referred for molecular testing. Three male infants were diagnosed with dystrophinopathy. Data from the first year of a two-year NBS pilot for DMD demonstrate the feasibility of NBS for DMD. The consortia approach was found to be a useful model, and the Newborn Screening Translational Research Network’s data tools played a key role in describing the NBS pilot findings and engaging stakeholders.

|
Scooped by
HAS-veille
September 16, 2022 4:03 AM
|
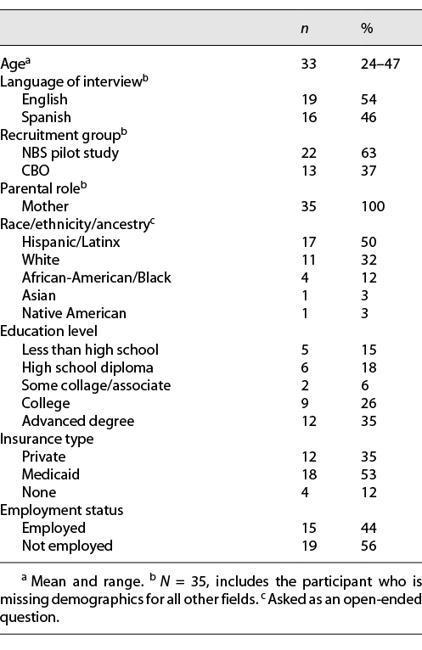
Objective: The aim of this study was to explore the parental views, attitudes, and preferences of expanded newborn screening (NBS) through genomic sequencing. Study Design: We conducted a semi-structured interview study with English and Spanish speaking mothers who had given birth within the USA in the past 5 years. The interviews explored opinions

|
Scooped by
HAS-veille
August 30, 2022 2:23 AM
|
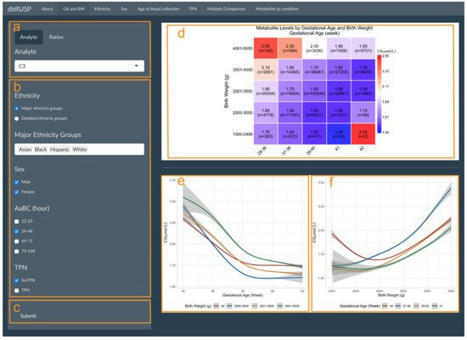
The Recommended Uniform Screening Panel (RUSP) contains more than forty metabolic disorders recommended for inclusion in universal newborn screening (NBS). Tandem-mass-spectrometry-based screening of metabolic analytes in dried blood spot samples identifies most affected newborns, along with a number of false positive results. Due to their influence on blood metabolite levels, continuous and categorical covariates such as gestational age, birth weight, age at blood collection, sex, parent-reported ethnicity, and parenteral nutrition status have been shown to reduce the accuracy of screening. Here, we developed a database and web-based tools (dbRUSP) for the analysis of 41 NBS metabolites and six variables for a cohort of 500,539 screen-negative newborns reported by the California NBS program. The interactive database, built using the R shiny package, contains separate modules to study the influence of single variables and joint effects of multiple variables on metabolite levels. Users can input an individual’s variables to obtain metabolite level reference ranges and utilize dbRUSP to select new candidate markers for the detection of metabolic conditions. The open-source format facilitates the development of data mining algorithms that incorporate the influence of covariates on metabolism to increase accuracy in genetic disease screening.

|
Scooped by
HAS-veille
August 19, 2022 1:27 AM
|
Introduction The goal of newborn bloodspot screening (NBS) is the early detection of treatable disorders in newborns to offer early intervention. Worldwide, the number of conditions screened for is expanding, which might affect public acceptance. In the Netherlands, participation is high (>99%), but little is known about how parents perceive NBS. This study assessed parents’ views on accepting, declining and expanding NBS. Methods A total of 804 of 6051 (13%) invited parents who participated in NBS in the Netherlands during the last two weeks of December 2019, and 48 of 1162 (4%) invited parents who declined participation in NBS in 2019 and 2020, completed a questionnaire. Results The most important reason for parents to participate in NBS was to prevent health complaints, whereas the most important reason to decline NBS was parents’ viewpoint on life and the belief that the heel prick would be painful for the child. Compared to NBS participants, respondents who declined NBS were more actively religious, considered alternative medicine or lifestyle more important, were less inclined to vaccinate their child for infectious diseases, and reported more doubt about NBS participation (all differences p < .001). Informed choice was lower among respondents who declined NBS (44%) compared to participants in NBS (83%, p < .001), mostly due to insufficient knowledge. Of the NBS participants, 95% were positive about NBS expansion. Most NBS participants agreed to include conditions that could unintentionally reveal a diagnosis in the mother instead of the child (86%) or a condition that may not cause symptoms until later in the child’s life (84%). Conclusion Most participants made an informed decision to participate in NBS and are positive about screening for more conditions. Insights into parents’ views on (non-)participation and expansion of NBS can help to ensure that NBS suits the population needs while safeguarding ethical principles for screening.

|
Scooped by
HAS-veille
August 10, 2022 4:22 AM
|
Duchenne muscular dystrophy is the most common form of muscular dystrophy diagnosed in childhood but is not routinely screened for prenatally or at birth in the United States. We sought t

|
Scooped by
HAS-veille
August 9, 2022 3:42 AM
|
Rapid advances in genomic technologies to screen, diagnose, and treat newborns will significantly increase the number of conditions in newborn screening (NBS). We previously identified four factors that delay and/or complicate NBS expansion: 1) variability in screening panels persists; 2) the short duration of pilots limits information about interventions and health outcomes; 3) recent recommended uniform screening panel (RUSP) additions are expanding the definition of NBS; and 4) the RUSP nomination and evidence review process has capacity constraints. In this paper, we developed a use case for each factor and suggested how model(s) could be used to evaluate changes and improvements. The literature on models was reviewed from a range of disciplines including system sciences, management, artificial intelligence, and machine learning. The results from our analysis highlighted that there is at least one model which could be applied to each of the four factors that has delayed and/or complicate NBS expansion. In conclusion, our paper supports the use of modeling to address the four challenges in the expansion of NBS.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...