Your new post is loading...
 Your new post is loading...

|
Scooped by
HAS-veille
March 11, 2019 9:23 AM
|
To assess the performance of a standardized, age-based metric for scoring clinical actionability to evaluate conditions for inclusion in newborn scree…

|
Scooped by
HAS-veille
March 7, 2019 11:45 AM
|
Abstract Newborn screening has evolved since its introduction in 1963. The disorders that are being screened for continue to evolve as new treatments and new technologies advance. In this review, the authors discuss the current state of newborn screening in the United States, including the disorders currently being screened for and how newborn screening is performed. They also discuss the special considerations and limitations of newborn screening in sick and premature infants and as well as some ethical issues related to newborn screening. Finally, new disorders being considered for testing and new technologies that may be used in the future of newborn screening are discussed.

|
Scooped by
HAS-veille
February 19, 2019 3:07 AM
|

|
Scooped by
HAS-veille
February 13, 2019 8:40 AM
|

|
Scooped by
HAS-veille
February 7, 2019 9:52 AM
|
Fragile X testing in newborns will now be available in North Carolina by way of a new study led by RTI International called Early Check.

|
Scooped by
HAS-veille
January 30, 2019 5:54 AM
|
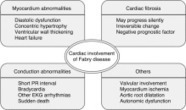
Fabry disease (FD) is an X-linked lysosomal storage disease and is the result of mutation in the α-Galactosidase A gene; such mutations cause a defici…

|
Scooped by
HAS-veille
January 24, 2019 7:13 AM
|
Sickle cell disease (SCD) is a severe inherited blood disorder associated with significant morbidity and mortality in early childhood. Since simple interventions are available to prevent earl

|
Scooped by
HAS-veille
January 22, 2019 10:44 AM
|

|
Scooped by
HAS-veille
January 11, 2019 7:46 AM
|

|
Scooped by
HAS-veille
January 10, 2019 8:20 AM
|
Performance of the Four-Plex Tandem Mass Spectrometry Lysosomal Storage Disease Newborn Screening Test: The Necessity of Adding a 2nd Tier Test for Pompe Disease by Shu-Chuan Chiang, Pin-Wen Chen, Wuh-Liang Hwu, An-Ju Lee, Li-Chu Chen, Ni-Chung Lee, Li-Yan Chiou and Yin-Hsiu Chien Int. J. Neonatal Screen. 2018, 4(4), 41; https://doi.org/10.3390/ijns4040041 Published: 18 December 2018 PDF Full-text (663 KB) | HTML Full-text | XML Full-text Newborn Screening for Lysosomal Storage Diseases: Methodologies, Screen Positive Rates, Normalization of Datasets, Second-Tier Tests, and Post-Analysis Tools by Michael H. Gelb Int. J. Neonatal Screen. 2018, 4(3), 23; https://doi.org/10.3390/ijns4030023 Published: 9 July 2018 Cited by 1 | PDF Full-text (244 KB) | HTML Full-text | XML Full-text Newborn Screening for Lysosomal Storage Disorders: Methodologies for Measurement of Enzymatic Activities in Dried Blood Spots by Michael H. Gelb, Zoltan Lukacs, Enzo Ranieri and Peter C. J. I. Schielen Int. J. Neonatal Screen. 2019, 5(1), 1; https://doi.org/10.3390/ijns5010001 Published: 21 December 2018 PDF Full-text (593 KB) | HTML Full-text | XML Full-text | Supplementary Files Current State of the Art of Newborn Screening for Lysosomal Storage Disorders by David S. Millington and Deeksha S. Bali Int. J. Neonatal Screen. 2018, 4(3), 24; https://doi.org/10.3390/ijns4030024 Published: 18 July 2018 PDF Full-text (463 KB) | HTML Full-text | XML Full-text Newborn Screening for Lysosomal Disease: Mission Creep and a Taste of Things to Come? by Bridget Wilcken Int. J. Neonatal Screen. 2018, 4(3), 21; https://doi.org/10.3390/ijns4030021 Published: 27 June 2018 PDF Full-text (173 KB) | HTML Full-text | XML Full-text

|
Scooped by
HAS-veille
January 10, 2019 8:11 AM
|
Sickle cell disease (SCD) and other hemoglobinopathies are a major health concern with a high burden of disease worldwide. Since the implementation of newborn screening (NBS) for SCD and other hemoglobinopathies in several regions of the world, technical progress of laboratory methods was achieved. This short review aims to summarize the current practice of classical laboratory methods for the detection of SCD and other hemoglobinopathies. This includes the newborn screening technologies of high-performance liquid chromatography (HPLC), capillary electrophoresis (CE), and isoelectric focusing (IEF).

|
Scooped by
HAS-veille
January 10, 2019 8:07 AM
|
Sickle cell disease (SCD) encompasses a group of inherited red cell disorders characterized by an abnormal hemoglobin, Hb S. The most common forms of SCD in the United States and Canada are identified through universal newborn screening (NBS) programs. Now carried out in all fifty U.S. states and 8 Canadian provinces, NBS for SCD represents one of the major public health advances in North America. The current status of NBS programs for hemoglobinopathies and the screening techniques employed in many regions worldwide reflect in large part the U.S. and Canadian experiences. Although the structure, screening algorithms and laboratory procedures, as well as reporting and follow up, vary between NBS programs, the overall workflow is similar. The current review summarized the historical background, current approaches, and methods used to screen newborns for SCD in the United States and Canada.

|
Scooped by
HAS-veille
January 10, 2019 8:04 AM
|
Glucose-6-phosphate dehydrogenase (G6PD) deficiency, an X-linked genetic disorder, is associated with increased risk of jaundice and kernicterus at birth. G6PD deficiency can manifest later in life as severe hemolysis, when the individual is exposed to oxidative agents that range from foods such as fava beans, to diseases such as typhoid, to medications such as dapsone, to the curative drugs for Plasmodium (P.) vivax malaria, primaquine and tafenoquine. While routine testing at birth for G6PD deficiency is recommended by the World Health Organization for populations with greater than 5% prevalence of G6PD deficiency and to inform P. vivax case management using primaquine, testing coverage is extremely low. Test coverage is low due to the need to prioritize newborn interventions and the complexity of currently available G6PD tests, especially those used to inform malaria case management. More affordable, accurate, point-of-care (POC) tests for G6PD deficiency are emerging that create an opportunity to extend testing to populations that do not have access to high throughput screening services. Some of these tests are quantitative, which provides an opportunity to address the gender disparity created by the currently available POC qualitative tests that misclassify females with intermediate G6PD activity as normal. In populations where the epidemiology for G6PD deficiency and P. vivax overlap, screening for G6PD deficiency at birth to inform care of the newborn can also be used to inform malaria case management over their lifetime.
|

|
Scooped by
HAS-veille
March 11, 2019 9:21 AM
|
In Germany, screening for cystic fibrosis (CF) is part of the newborn screening since September 2016. The risk of psychological harm due to false-positive screening results is a longstandin

|
Scooped by
HAS-veille
March 7, 2019 11:33 AM
|

|
Scooped by
HAS-veille
February 13, 2019 8:49 AM
|
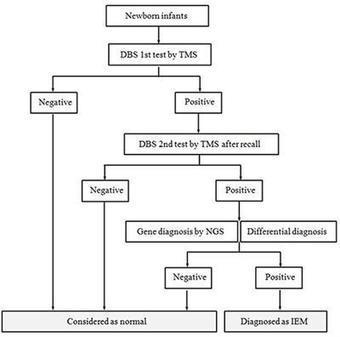
Newborn screening and changing face of inborn errors of metabolism in the United States

|
Scooped by
HAS-veille
February 7, 2019 9:54 AM
|
Decade-long campaign by the Immune Deficiency Foundation and numerous partners realizes goal of testing every child in the United States for this life-threatening disease Challenging work remains to address gaps in testing and care for people with SCID

|
Scooped by
HAS-veille
February 5, 2019 9:27 AM
|
Background In 2002, a nationwide screening for congenital adrenal hyperplasia (CAH) was introduced in the Netherlands. The aim of our study is to evaluate the validity of the neonatal screening for CAH and to assess how many newborns with salt-wasting (SW) CAH have already been clinically diagnosed before the screening result was known.
Conclusion The Dutch neonatal screening has an excellent sensitivity and high specificity. Both boys and girls can benefit from neonatal screening.

|
Scooped by
HAS-veille
January 28, 2019 9:04 AM
|
OBJECTIVES: Newborn screening for severe combined immunodeficiency (SCID) was instituted in California in 2010. In the ensuing 6.5 years, 3 252 156 infants in the state had DNA from dried blood spots assayed for T-cell receptor excision circles (TRECs). Abnormal TREC results were followed-up with liquid blood testing for T-cell abnormalities. We report the performance of the SCID screening program and the outcomes of infants who were identified.
CONCLUSIONS: Population-based TREC testing, although unable to detect immune defects in which T cells are present at birth, is effective for identifying SCID and clinically important TCL with high sensitivity and specificity. The experience in California supports the rapid, widespread adoption of SCID newborn screening.

|
Scooped by
HAS-veille
January 22, 2019 10:54 AM
|

|
Scooped by
HAS-veille
January 11, 2019 7:56 AM
|
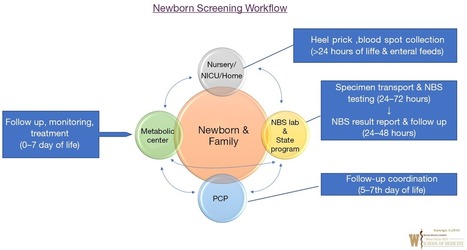
The Future of Newborn Screening: Why and How Partnerships Will Be Needed for Success North Carolina Medical Journal January-February 2019 80:28-31; doi:10.18043/ncm.80.1.28 What is Newborn Screening? North Carolina Medical Journal January-February 2019 80:32-36; doi:10.18043/ncm.80.1.32 Newborn Screening Follow-up North Carolina Medical Journal January-February 2019 80:37-41; doi:10.18043/ncm.80.1.37 The Role of the Genetic Counselor in Newborn Screening North Carolina Medical Journal January-February 2019 80:39-40; doi:10.18043/ncm.80.1.39 Newborn Screening Policy Decisions: Adding Conditions North Carolina Medical Journal January-February 2019 80:42-44; doi:10.18043/ncm.80.1.42 Recognizing 50 Years of Innovative Newborn Screening in North Carolina North Carolina Medical Journal January-February 2019 80:45-48; doi:10.18043/ncm.80.1.45 The Role of Technology in Newborn Screening North Carolina Medical Journal January-February 2019 80:49-53; doi:10.18043/ncm.80.1.49 Lessons Learned from Newborn Screening in Pilot Studies North Carolina Medical Journal January-February 2019 80:54-58; doi:10.18043/ncm.80.1.54

|
Scooped by
HAS-veille
January 10, 2019 8:37 AM
|

|
Scooped by
HAS-veille
January 10, 2019 8:13 AM
|
The implementation of newborn screening for severe combined immunodeficiency (SCID) in the Netherlands is a multifaceted process in which several parties are involved. The Dutch Ministry of Health adopted the advice of the Dutch Health Council to include SCID in the Dutch newborn screening program in 2015. As newborn screening for SCID is executed with a new, relatively expensive assay for the Dutch screening laboratory, an implementation pilot study is deemed instrumental for successful implementation. A feasibility study was performed in which the practicalities and preconditions of expanding the newborn screening program were defined. Cost-effectiveness analysis (CEA) indicated that SCID screening in the Netherlands might be cost-effective, recognizing that there are still many uncertainties in the variables underlying the CEA. Data and experience of the pilot study should provide better estimates of these parameters, thus enabling the actualization of CEA results. Prior to the implementation pilot study, a comparison study of two commercially available SCID screening assays was performed. A prospective implementation pilot study or so-called SONNET study (SCID screening research in the Netherlands with TRECs) started in April 2018 and allows the screening for SCID of all newborns in three provinces of the Netherlands for one year. Based on the results of the SONNET study, the Dutch Ministry of Health will make a final decision about national implementation of newborn screening for SCID in the Netherlands.

|
Scooped by
HAS-veille
January 10, 2019 8:10 AM
|
Our previous results reported that compared to sickle cell patients who were not screened at birth, those who benefited from it had a lower incidence of a first bacteremia and a reduced number and days of hospitalizations. In this context, this article reviews the Belgian experience on neonatal screening for sickle cell disease (SCD). It gives an update on the two regional neonatal screening programs for SCD in Belgium and their impact on initiatives to improve clinical care for sickle cell patients. Neonatal screening in Brussels and Liège Regions began in 1994 and 2002, respectively. Compiled results for the 2009 to 2017 period demonstrated a birth prevalence of sickle cell disorder above 1:2000. In parallel, to improve clinical care, (1) a committee of health care providers dedicated to non-malignant hematological diseases has been created within the Belgian Haematology Society; (2) a clinical registry was implemented in 2008 and has been updated in 2018; (3) a plan of action has been proposed to the Belgian national health authority. To date, neonatal screening is not integrated into the respective Belgian regional neonatal screening programs, the ongoing initiatives in Brussels and Liège Regions are not any further funded and better management of the disease through the implementation of specific actions is not yet perceived as a public health priority in Belgium.

|
Scooped by
HAS-veille
January 10, 2019 8:06 AM
|
There is a growing demand for newborn sickle cell disease screening globally. Historically techniques have relied on the separation of intact haemoglobin tetramers using electrophoretic or liquid chromatography techniques. These techniques also identify haemoglobin variants of no clinical significance. Specific electrospray ionization-mass spectrometry-mass spectrometry techniques to analyse targeted peptides formed after digestion of the haemoglobin with trypsin were reported in 2005. Since this time the method has been further developed and adopted in several European countries. It is estimated that more than one million babies have been screened with no false-negative cases reported. This review reports on the current use of the technique and reviews the related publications.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...