Your new post is loading...
 Your new post is loading...

|
Scooped by
HAS-veille
September 22, 2023 3:28 AM
|
The collection of dried blood spots (DBS) facilitates newborn screening for a variety of rare, but very serious conditions in healthcare systems around the world. Sub-punches of varying sizes (1.5–6 mm) can be taken from DBS specimens to use as inputs for a range of biochemical assays. Advances in DNA sequencing workflows allow whole-genome sequencing (WGS) libraries to be generated directly from inputs such as peripheral blood, saliva, and DBS. We compared WGS metrics obtained from libraries generated directly from DBS to those generated from DNA extracted from peripheral blood, the standard input for this type of assay. We explored the flexibility of DBS as an input for WGS by altering the punch number and size as inputs to the assay. We showed that WGS libraries can be successfully generated from a variety of DBS inputs, including a single 3 mm or 6 mm diameter punch, with equivalent data quality observed across a number of key metrics of importance in the detection of gene variants. We observed no difference in the performance of DBS and peripheral-blood-extracted DNA in the detection of likely pathogenic gene variants in samples taken from individuals with cystic fibrosis or phenylketonuria. WGS can be performed directly from DBS and is a powerful method for the rapid discovery of clinically relevant, disease-causing gene variants.

|
Scooped by
HAS-veille
September 19, 2023 2:15 AM
|
Studies of genomic newborn screening are highly skewed towards populations in high-income countries. The evidence generated by these studies will be similarly biased and is likely to lead to disparate global implementation. Studies inclusive of historically under-represented populations are needed for equitable global access to genomic newborn screening. In this Comment, Ahmad Abou Tayoun advocates for studies inclusive of historically under-represented populations to ensure equitable global access to genomic newborn screening.

|
Scooped by
HAS-veille
September 15, 2023 7:28 AM
|
Introduction: The Portuguese Neonatal Screening Programme (PNSP) identifies patients with rare diseases through nationwide screening. Currently, 27 diseases are diagnosed, amongst which are 24 Inborn Errors of Metabolism (IEM), covering approximately 100% of neonates (1). In 2004, the national laboratory implemented a new screening method, tandem mass spectrometry (MS/MS) to test for amino acids and acylcarnitines. This new protocol revolutionized the PNSP and allowed for the analysis of an increased number of IEM, with clear improvements in treatment timings and clinical outcomes (2).
Conclusion: This data shows the molecular epidemiology of patients with confirmed IEM diagnosis identified by neonatal screening. Some diseases out of the scope of the PNSP were also detected as a differential diagnosis after biochemical suspicion in the dried blood spot sample. The retrospective analysis of the PNSP allows for an overview of 18 years of achievements accomplished by the national screening for IEM since MS/MS was implemented. For some pathologies with low incidence, it’s difficult to trace a discernible pattern. However, presenting de novo mutations for these diseases might provide insights on how to approach different phenotypes. The aim of this work is to establish the molecular epidemiology of metabolic diseases screened.

|
Scooped by
HAS-veille
September 15, 2023 4:06 AM
|
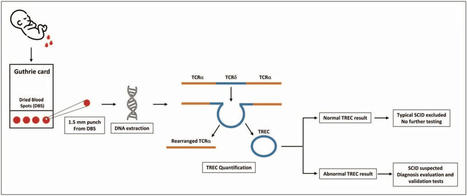
SCID marked the pioneering inborn error of immunity (IEI) to undergo NBS, a milestone achieved 15 years ago through the enumeration of T-cell receptor excision circles (TRECs) extracted from Guthrie cards. This breakthrough has revolutionized our approach to SCID, enabling not only presymptomatic identification and prompt treatments (including hematopoietic stem cell transplantation), but also enhancing our comprehension of the global epidemiology of SCID.
NBS is continuing to evolve with the advent of novel diagnostic technologies and treatments. Following the successful implementation of SCID-NBS programs, a call for the early identification of additional IEIs is the next step, encompassing a broader spectrum of IEIs, facilitating early diagnoses, and preventing morbidity an

|
Scooped by
HAS-veille
September 8, 2023 1:52 AM
|
Newborn screening (NBS) programmes for severe combined immunodeficiency (SCID) facilitate
early SCID diagnosis and promote early treatment with haematopoietic stem cell transplantation,
resulting in improved clinical outcomes. Infants with congenital athymia are also
identified through NBS due to severe T-cell lymphopaenia. With the expanding introduction
of NBS programmes, referrals of athymic patients for treatment with thymus transplantation
have recently increased at Great Ormond Street Hospital (GOSH), London, United Kingdom.

|
Scooped by
HAS-veille
September 4, 2023 1:21 AM
|
Newborn screening is a crucial global public health initiative, with a primary aim to identify congenital disorders that could lead to significant morbidity and mortality if left untreated. However, the scope of traditional newborn screening methods is limited, detecting only a finite numbe

|
Scooped by
HAS-veille
September 1, 2023 8:58 AM
|

|
Scooped by
HAS-veille
August 28, 2023 1:37 AM
|
Long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) and mitochondrial trifunctional protein (MTP) deficiencies are rare fatal disorders of fatty acid β-oxidation with no apparent genotype–phenotype correlation. The measurement of acylcarnitines by MS/MS is a current diagnostic workup in these disorders. Nevertheless, false-positive and false-negative results have been reported, highlighting a necessity for more sensitive and specific biomarkers. This study included 54 patients with LCHAD/MTP deficiency that has been confirmed by biochemical and molecular methods. The analysis of acylcarnitines in dried blood spots was performed using ESI-MS/MS. The established “HADHA ratio” = (C16OH + C18OH + C18:1OH)/C0 was significantly elevated in all 54 affected individuals in comparison to the control group. Apart from 54 LCHAD deficiency patients, the “HADHA ratio” was calculated in 19 patients with very-long-chain acyl-CoA dehydrogenase (VLCAD) deficiency. As VLCAD-deficient patients did not show increased “HADHA ratio”, the results emphasized the high specificity of this new ratio. Therefore, the “HADHA ratio” was shown to be instrumental in improving the overall performance of MS/MS-based analysis of acylcarnitine levels in the diagnostics of LCHAD/MTP deficiencies. The ratio was demonstrated to increase the sensitivity and specificity of this method and reduce the chances of false-negative results.

|
Scooped by
HAS-veille
August 28, 2023 1:34 AM
|
The use of next-generation sequencing technologies such as genomic sequencing in newborn screening (NBS) could enable the detection of a broader range of conditions. We explored parental preferences and attitudes towards screening for conditions for which varying types of treatment exist with a cross-sectional survey completed by 100 parents of newborns who received NBS in Ontario, Canada. The survey included four vignettes illustrative of hypothetical screening targets, followed by questions assessing parental attitudes. Chi-square tests were used to compare frequency distributions of preferences. Results show that most parents supported NBS for conditions for which only supportive interventions are available, but to a significantly lesser degree than those with disease-specific treatments (99% vs. 82–87%, p ≤ 0.01). For conditions without an effective treatment, the type of supportive care and age of onset of the condition did not significantly alter parent perceptions of risks and benefits. Parents are interested in expanded NBS for conditions with only supportive interventions in childhood, despite lower levels of perceived benefit for the child and greater anticipated anxiety from screen-positive results. These preferences suggest that the expansion of NBS may require ongoing deliberation of perceived benefits and risks and enhanced approaches to education, consent, and support.

|
Scooped by
HAS-veille
August 22, 2023 2:52 AM
|
Aim
We aimed to familiarise clinicians with the terms cystic fibrosis transmembrane conductance regulator related metabolic syndrome (CRMS) and cystic fibrosis screen positive inconclusiv

|
Scooped by
HAS-veille
August 21, 2023 6:14 AM
|
This Commentary summarizes what the author has learned in 46 years of research on newborn screening (NBS) for cystic fibrosis (CF) combined with healt…

|
Scooped by
HAS-veille
August 9, 2023 4:30 AM
|
Objective: Until November 2019 in Belgium, dried blood spot sampling (DBS) was performed between 72 and 120 hours of life, when a majority of newborns had already been discharged from the maternity. In November 2019, the policy for newborn screening in South Belgium changed to allow sampling as soon as 48 hours of life, with the objective to accelerate the process and to allow more sampling during the hospital stay. Our objective was to evaluate the impact of this policy modification and, in particular, to assess the effectiveness of screening for hypothyroidism based on sampling before or after 72 hours of life, as well as to compare the effectiveness of DBS collection before discharge or at home. Methods: This retrospective study included live births ≥ 37 weeks of gestation, screened by the Université Libre de Bruxelles (ULB) Newborn Screening Center between January 2019 and December 2021. In order to evaluate the efficiency of early sampling, we compared TSH results for screening <72 hours and screening ≥72 hours. We also compared TSH results of DBS performed before discharge with those performed at home. Results: A total of 53,794 newborns were included. The results of 24,816 healthy newborns screened before 72 hours of life and of 28,978 healthy newborns screened between 72 and 144 hours of life were compared. The median TSH level was similar (1.50 mU/L and 1.20 mU/L, respectively). The percentage of false positives was similar (0.08% and 0.07%, respectively). Earlier sampling, before 72 hours, allowed treatment of positive cases at 6 days rather than 8.5 days. DBS sampling at home resulted in longer delay for transferring the sample to the laboratory (a median of 3.0 days for hospital sampling vs 5.0 days for home sampling). A poorer quality of home blood sampling was observed, with 0.27% unusable samples compared with 0.06 % unusable samples for hospital sampling (p <0.001). Conclusions: In term newborns, TSH screening before discharge, as early as 48 hours of life, is a valid strategy. It allows earlier treatment of positive cases, does not increase the percentage of false positives and results in fewer unusable samples.

|
Scooped by
HAS-veille
August 8, 2023 5:46 AM
|
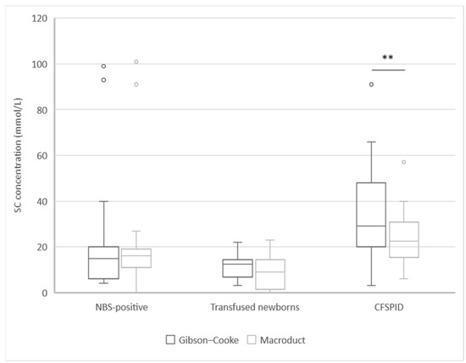
The sweat test (ST) is the current diagnostic gold standard for cystic fibrosis (CF). Many CF centres have switched from the Gibson–Cooke method to the Macroduct system-based method. We used these methods simultaneously to compare CF screening outcomes. STs using both methods were performed simultaneously between March and December 2022 at CF Centre in Florence. We included newborns who underwent newborn bloodspot screening (NBS), newborns undergoing transfusion immediately after birth, and children with CF screen-positive, inconclusive diagnosis (CFSPID). We assessed 72 subjects (median age 4.4 months; range 0–76.7): 30 (41.7%) NBS-positive, 18 (25.0%) newborns who underwent transfusion, and 24 (33.3%) children with CFSPID. No significant differences were found between valid sample numbers, by patient ages and groups (p = 0.10) and between chloride concentrations (p = 0.13), except for sweat chloride (SC) measured by the Gibson–Cooke and Macroduct methods in CFSPID group (29.0, IQR: 20.0–48.0 and 22.5, IQR: 15.5–30.8, respectively; p = 0.01). The Macroduct and Gibson–Cooke methods showed substantial agreement with the SC values, except for CFSPID, whose result may depend on the method of sweat collection. In case of invalid values with Macroduct, the test should be repeated with Gibson–Cooke method.
|

|
Scooped by
HAS-veille
September 20, 2023 7:57 AM
|
Newborn screening (NBS) began in the early 1960s with screening for phenylketonuria on blood collected on filter paper. The number of conditions included in NBS programs expanded significantly with the adoption of tandem mass spectrometry. The recommended uniform screening panel provides national guidance and has reduced state variability. Universality and uniformity have been supported to promote equity. Recently, a number of researchers have suggested expanding NBS to include genomic sequencing to identify all genetic disorders in newborns. This has been specifically suggested for genes that increase the risk for neurodevelopmental disorders (NDDs), with the presumption that early identification in the newborn period would reduce disabilities. We offer arguments to show that genomic sequencing of newborns for NDDs risks exacerbating disparities. First, the diagnosis of NDD requires clinical expertise, and both genetic and neurodevelopmental expertise are in short supply, leading to disparities in access to timely follow-up. Second, therapies for children with NDDs are insufficient to meet their needs. Increasing early identification for those at risk who may never manifest developmental delays could shift limited resources to those children whose parents are more poised to advocate, worsening disparities in access to services. Rather, we suggest an alternative: genomic sequencing of all children with diagnosed NDDs. This focused strategy would have the potential to target genomic sequencing at children who manifest NDDs across diverse populations which could better improve our understanding of contributory genes to NDDs.

|
Scooped by
HAS-veille
September 18, 2023 2:50 AM
|
Dutch newborn screening (NBS) for Cystic Fibrosis (CF) introduced in 2011 showed a sensitivity of 90% and a positive predictive value (PPV) of 63%. We…

|
Scooped by
HAS-veille
September 15, 2023 7:27 AM
|
Introduction: Newborn screening (NBS) in Portugal is a significant public health measure to provide early detection for specific disorders so that early treatment is possible. Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disorder that causes degeneration of anterior horn cells in the human spinal cord and subsequent loss of motor neurons. Its incidence is estimated in 1.6000-11.800 live births. A pilot study on 100.000 newborns is being carried out at the neonatal screening laboratory with the aim of determining the specificity, sensitivity, and feasibility of the SMA screening at the NBS laboratory in Portugal.
Conclusion: Early diagnosis and intervention are important for SMA treatment to be effective; the treatment should be started at the pre-symptomatic stage of SMA. Thus, newborn screening for SMA is strongly recommended. Currently, targeted therapies for SMA are available, and attempts are being made worldwide to include SMA screening in newborns.

|
Scooped by
HAS-veille
September 11, 2023 3:55 AM
|
Citrin deficiency is an autosomal recessive disorder caused by a defect of citrin resulting from mutations in the SLC25A13 gene. Intrahepatic cholestasis and various metabolic abnormalities

|
Scooped by
HAS-veille
September 4, 2023 1:28 AM
|
Newborn screening (NBS) for X-linked adrenoleukodystrophy (ALD) can identify affected individuals before the onset of life-threatening manifestations. Some countries have decided to only screen boys (sex-specific screening). This study investigates the attitudes of individuals with ALD towards sex-specific NBS for ALD. A questionnaire was sent to all patients in the Dutch ALD cohort. Invitees were asked who they thought should be screened for ALD: only boys, both boys and girls or neither. The motives and background characteristics of respondents were compared between screening preferences. Out of 108 invitees, 66 participants (61%), 38 men and 28 women, participated in this study. The majority (n = 53, 80%) favored screening both newborn boys and girls for ALD, while 20% preferred boys only. None of the respondents felt that newborns should not be screened for ALD. There were no differences in the background characteristics of the respondents between screening preferences. Our study revealed a diverse range of motivations underlying respondents’ screening preferences. This study is one of the first to investigate the attitudes of patients towards sex-specific screening for ALD. The outcomes of this study can offer insights to stakeholders engaged in the implementation of NBS programs. ALD patients are important stakeholders who can provide valuable input in this process.

|
Scooped by
HAS-veille
September 4, 2023 1:19 AM
|
This cohort study of Chinese newborns examines the outcomes of gene panel sequencing as a first-tier newborn screening test supplementing biochemical screening for monogenic disorders.

|
Scooped by
HAS-veille
August 28, 2023 7:05 AM
|
Over the last decade, treatment of spinal muscular atrophy (SMA) has become a paradigm of the importance of early and accurate diagnosis and prompt treatment. Three different therapeuti

|
Scooped by
HAS-veille
August 28, 2023 1:35 AM
|
As of December 2009, cystic fibrosis (CF) newborn screening (NBS) is performed in all 50 US states and the District of Columbia. Widespread implementation of CF newborn screening (CFNBS) in the US and internationally has brought about new and varied challenges. Immunoreactive trypsinogen (IRT) remains the first, albeit imperfect, biomarker used universally in the screening process. Advances in genetic testing have provided an opportunity for newborn screening programs to add CFTR sequencing tiers to their algorithms. This in turn will enable earlier identification of babies with CF and improve longer-term outcomes through prompt treatment and intervention. CFTR sequencing has led to the ability to identify infants with CF from diverse ethnic and racial backgrounds more equitably while also identifying an increasing proportion of infants with inconclusive diagnoses. Using the evolution of the New York State CF newborn screening program as a guide, this review outlines the basic steps in a universal CF newborn screening program, considers how to reduce bias, highlights challenges, offers guidance to address these challenges and provides recommendations for future consideration.

|
Scooped by
HAS-veille
August 22, 2023 2:56 AM
|
Analytical and therapeutic innovations led to a continuous but variable extension of newborn screening (NBS) programs worldwide. Every extension requires a careful evaluation of feasibility

|
Scooped by
HAS-veille
August 21, 2023 7:57 AM
|
Severe combined immunodeficiency (SCID) is one of the most severe forms of inborn errors of immunity (IEI), affecting both cellular and humoral immunity. Without curative treatment such a

|
Scooped by
HAS-veille
August 21, 2023 6:11 AM
|
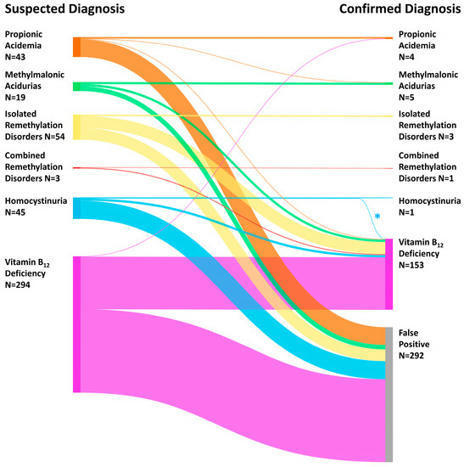
Newborn screening (NBS) programs are effective measures of secondary prevention and have been successively extended. We aimed to evaluate NBS for methylmalonic acidurias, propionic acidemia, homocystinuria, remethylation disorders and neonatal vitamin B12 deficiency, and report on the identification of cofactor-responsive disease variants. This evaluation of the previously established combined multiple-tier NBS algorithm is part of the prospective pilot study “NGS2025” from August 2016 to September 2022. In 548,707 newborns, the combined algorithm was applied and led to positive NBS results in 458 of them. Overall, 166 newborns (prevalence 1: 3305) were confirmed (positive predictive value: 0.36); specifically, methylmalonic acidurias (N = 5), propionic acidemia (N = 4), remethylation disorders (N = 4), cystathionine beta-synthase (CBS) deficiency (N = 1) and neonatal vitamin B12 deficiency (N = 153). The majority of the identified newborns were asymptomatic at the time of the first NBS report (total: 161/166, inherited metabolic diseases: 9/14, vitamin B12 deficiency: 153/153). Three individuals were cofactor-responsive (methylmalonic acidurias: 2, CBS deficiency: 1), and could be treated by vitamin B12, vitamin B6 respectively, only. In conclusion, the combined NBS algorithm is technically feasible, allows the identification of attenuated and severe disease courses and can be considered to be evaluated for inclusion in national NBS panels.

|
Scooped by
HAS-veille
August 8, 2023 5:47 AM
|
Spinal muscular atrophy (SMA) is a progressive neuromuscular disease caused by biallelic pathogenic/likely pathogenic variants of the survival motor neuron 1 (SMN1) gene. Early diagnosis via newborn screening (NBS) and pre-symptomatic treatment are essential to optimize health outcomes for affected individuals. We developed a multiplex quantitative polymerase chain reaction (qPCR) assay using dried blood spot (DBS) samples for the detection of homozygous absence of exon 7 of the SMN1 gene. Newborns who screened positive were seen urgently for clinical evaluation. Confirmatory testing by multiplex ligation-dependent probe amplification (MLPA) revealed SMN1 and SMN2 gene copy numbers. Six newborns had abnormal screen results among 47,005 newborns screened during the first year and five were subsequently confirmed to have SMA. Four of the infants received SMN1 gene replacement therapy under 30 days of age. One infant received an SMN2 splicing modulator due to high maternally transferred AAV9 neutralizing antibodies (NAb), followed by gene therapy at 3 months of age when the NAb returned negative in the infant. Early data show that all five infants made excellent developmental progress. Based on one year of data, the incidence of SMA in Alberta was estimated to be 1 per 9401 live births.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...