The authors show that early Th2 cell differentiation is driven via prolonged T–DC macro-clustering in lymph nodes and occurs in a skin site-specific manner
Follow, research and publish the best content
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account
Already have an account: Login
Teaching and Learning Immunology. Information you never would have searched for!
Curated by
Gilbert C FAURE
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
|
|




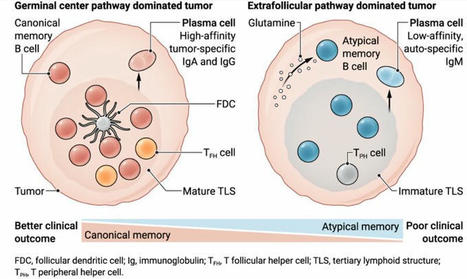








T helper 2 (Th2) responses protect against pathogens while also driving allergic inflammation, yet how large-scale Th2 responses are generated in tissue context remains unclear. Here, we used quantitative imaging to investigate early Th2 differentiation within lymph nodes (LNs) following cutaneous allergen administration. Contrary to current models, we observed extensive activation and “macro-clustering” of early Th2 cells with migratory type-2 dendritic cells (cDC2s), generating specialized Th2-promoting microenvironments. Macro-clustering was integrin-mediated and promoted localized cytokine exchange among T cells to reinforce differentiation, which contrasted the behavior during Th1 responses. Unexpectedly, formation of Th2 macro-clusters was dependent on the site of skin sensitization. Differences between sites were driven by divergent activation states of migratory cDC2 from different dermal tissues, with enhanced costimulatory molecule expression by cDC2 in Th2-generating LNs promoting prolonged T cell activation, macro-clustering, and cytokine sensing. Thus, the generation of dedicated Th2 priming microenvironments through enhanced costimulatory molecule signaling initiates Th2 responses in vivo and occurs in a skin site-specific manner.