Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
September 19, 2024 4:28 AM
|
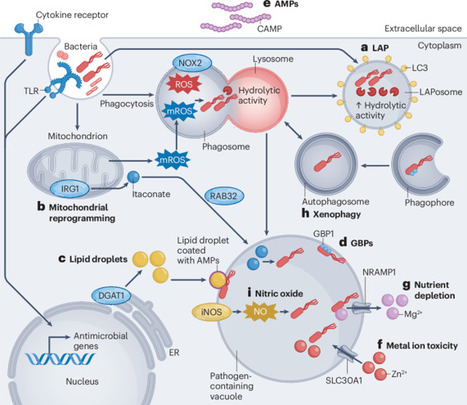
Macrophages destroy bacteria and other microorganisms through phagocytosis-coupled antimicrobial responses, such as the generation of reactive oxygen species and the delivery of hydrolytic enzymes from lysosomes to the phagosome. However, many intracellular bacteria subvert these responses, escaping to other cellular compartments to survive and/or replicate. Such bacterial subversion strategies are countered by a range of additional direct antibacterial responses that are switched on by pattern-recognition receptors and/or host-derived cytokines and other factors, often through inducible gene expression and/or metabolic reprogramming. Our understanding of these inducible antibacterial defence strategies in macrophages is rapidly evolving. In this Review, we provide an overview of the broad repertoire of antibacterial responses that can be engaged in macrophages, including LC3-associated phagocytosis, metabolic reprogramming and antimicrobial metabolites, lipid droplets, guanylate-binding proteins, antimicrobial peptides, metal ion toxicity, nutrient depletion, autophagy and nitric oxide production. We also highlight key inducers, signalling pathways and transcription factors involved in driving these different antibacterial responses. Finally, we discuss how a detailed understanding of the molecular mechanisms of antibacterial responses in macrophages might be exploited for developing host-directed therapies to combat antibiotic-resistant bacterial infections. Macrophages are innate immune sentinels providing frontline defence against infection. This Review describes the inducible mechanisms used by macrophages to kill bacterial pathogens and/or inhibit their growth and outlines how this knowledge might be exploited in the design of host-directed therapies.

|
Scooped by
Gilbert C FAURE
September 12, 2024 4:20 AM
|

|
Scooped by
Gilbert C FAURE
September 6, 2024 10:56 AM
|
Researchers at the Institut Pasteur in France have developed artificial “lymphoid organ-chips” that recreate much of the human immune system’s response to booster vaccines. The technology, described in an article to be published September 6 in the Journal of Experimental Medicine (JEM), could potentially be used to evaluate the likely effectiveness of new protein and mRNA-based booster vaccines for COVID-19 and other infectious diseases.

|
Scooped by
Gilbert C FAURE
August 12, 2024 4:04 AM
|
A team of researchers, led by the University of Melbourne’s Professor Laura Mackay, Laboratory Head and Theme Leader in Immunology at the Doherty Institute, has uncovered the mechanisms that govern the development of memory T cells in various parts of the body during infection.

|
Scooped by
Gilbert C FAURE
August 8, 2024 5:46 AM
|
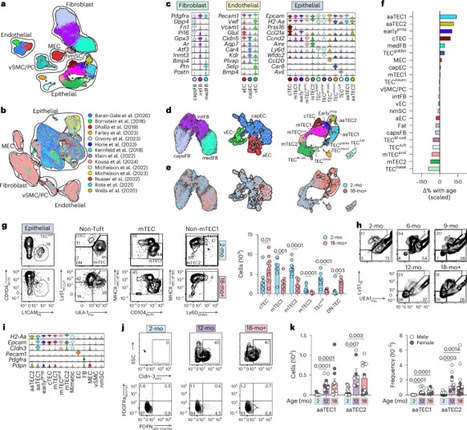
The thymus is essential for establishing adaptive immunity yet undergoes age-related involution that leads to compromised immune responsiveness. The thymus is also extremely sensitive to acute insult and although capable of regeneration, this capacity declines with age for unknown reasons. We applied single-cell and spatial transcriptomics, lineage-tracing and advanced imaging to define age-related changes in nonhematopoietic stromal cells and discovered the emergence of two atypical thymic epithelial cell (TEC) states. These age-associated TECs (aaTECs) formed high-density peri-medullary epithelial clusters that were devoid of thymocytes; an accretion of nonproductive thymic tissue that worsened with age, exhibited features of epithelial-to-mesenchymal transition and was associated with downregulation of FOXN1. Interaction analysis revealed that the emergence of aaTECs drew tonic signals from other functional TEC populations at baseline acting as a sink for TEC growth factors. Following acute injury, aaTECs expanded substantially, further perturbing trophic regeneration pathways and correlating with defective repair of the involuted thymus. These findings therefore define a unique feature of thymic involution linked to immune aging and could have implications for developing immune-boosting therapies in older individuals. Here the authors identify age-associated changes in the epithelial cell compartment of the thymus that form high-density nonproductive microenvironmental niches that contribute toward thymic involution and inhibit its repair following injury.

|
Scooped by
Gilbert C FAURE
July 11, 2024 4:21 AM
|
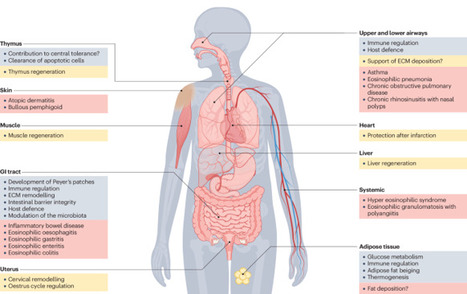
Eosinophils are bone marrow-derived granulocytes that are traditionally associated with type 2 immune responses, such as those that occur during parasite infections and allergy. Emerging evidence demonstrates the remarkable functional plasticity of this elusive cell type and its pleiotropic functions in diverse settings. Eosinophils broadly contribute to tissue homeostasis, host defence and immune regulation, predominantly at mucosal sites. The scope of their activities primarily reflects the breadth of their portfolio of secreted mediators, which range from cytotoxic cationic proteins and reactive oxygen species to multiple cytokines, chemokines and lipid mediators. Here, we comprehensively review basic eosinophil biology that is directly related to their activities in homeostasis, protective immunity, regeneration and cancer. We examine how dysregulation of these functions contributes to the physiopathology of a broad range of inflammatory diseases. Furthermore, we discuss recent findings regarding the tissue compartmentalization and adaptation of eosinophils, shedding light on the factors that likely drive their functional diversification within tissues. This Review by Arnold and Munitz discusses the diverse roles of eosinophils in the settings of tissue homeostasis, infection, allergy and cancer. The authors explain the molecular mechanisms that enable eosinophils to adapt to diverse tissue types and conditions, and they consider the therapeutic potential of eosinophil-depleting drugs in the clinic.

|
Scooped by
Gilbert C FAURE
July 11, 2024 3:47 AM
|
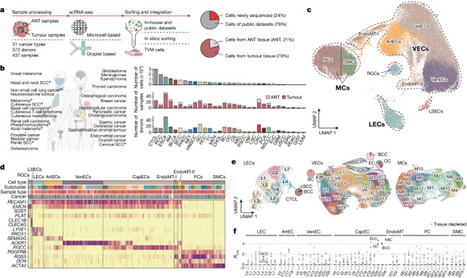
Tumours can obtain nutrients and oxygen required to progress and metastasize through the blood supply1. Inducing angiogenesis involves the sprouting of established vessel beds and their maturation into an organized network2,3. Here we generate a comprehensive atlas of tumour vasculature at single-cell resolution, encompassing approximately 200,000 cells from 372 donors representing 31 cancer types. Trajectory inference suggested that tumour angiogenesis was initiated from venous endothelial cells and extended towards arterial endothelial cells. As neovascularization elongates (through angiogenic stages SI, SII and SIII), APLN+ tip cells at the SI stage (APLN+ TipSI) advanced to TipSIII cells with increased Notch signalling. Meanwhile, stalk cells, following tip cells, transitioned from high chemokine expression to elevated TEK (also known as Tie2) expression. Moreover, APLN+ TipSI cells not only were associated with disease progression and poor prognosis but also hold promise for predicting response to anti-VEGF therapy. Lymphatic endothelial cells demonstrated two distinct differentiation lineages: one responsible for lymphangiogenesis and the other involved in antigen presentation. In pericytes, endoplasmic reticulum stress was associated with the proangiogenic BASP1+ matrix-producing pericytes. Furthermore, intercellular communication analysis showed that neovascular endothelial cells could shape an immunosuppressive microenvironment conducive to angiogenesis. This study depicts the complexity of tumour vasculature and has potential clinical significance for anti-angiogenic therapy. An atlas of tumour vasculature shows that tumour angiogenesis is initiated from venous endothelial cells and extended towards arterial endothelial cells.

|
Scooped by
Gilbert C FAURE
July 9, 2024 2:51 AM
|
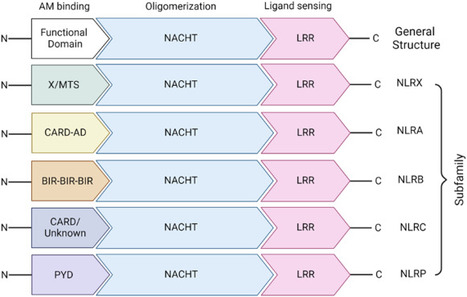
Proinflammatory cytokines and chemokines play a crucial role in regulating the inflammatory response, which is essential for the proper functioning of our immune system. When infections or threats to the body’s defense mechanisms are detected, the innate immune system takes the lead. However, an excessive inflammatory response can lead to the production of high concentrations of cytotoxic molecules, resulting in tissue damage. Inflammasomes are significant contributors to innate immunity, and one of the most extensively studied inflammasome complexes is NOD-like receptor 3 (NLRP3). NLRP3 has a wide range of recognition mechanisms that streamline immune activation and eliminate pathogens. These cytosolic multiprotein complexes are composed of effector, adaptor, and sensor proteins, which are crucial for identifying intracellular bacterial breakdown products and initiating an innate immune cascade. To understand the diverse behavior of NLRP3 activation and its significance in the development of lifestyle-related diseases, one must delve into the study of the immune response and apoptosis mediated by the release of proinflammatory cytokines. In this review, we briefly explore the immune response in the context of lifestyle associated disorders such as obesity, hyperlipidemia, diabetes, chronic respiratory disease, oral disease, and cardiovascular disease. NOD-like receptors (NLRs - proteins that help our immune system fight off harmful invaders) are vital for our health. Their function in T and B cells (types of white blood cells) is less clear. Scientists have found 22 kinds of NLRs in humans, which start different immune and inflammation responses. This study is a detailed review of NLRs, examining their structure, how they are activated, and their role in diseases like obesity, diabetes, and heart problems. It emphasizes that NLRs, particularly the NLRP3 inflammasome (a protein complex involved in inflammation), are key in lifestyle diseases by causing inflammation. The review proposes that focusing on NLRP3 could lead to new treatments for these diseases. This research is a big step in understanding how our natural immune system contributes to chronic diseases and offers potential for new treatments. Future research could further explore the complexities of NLRs and their potential as treatment targets. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.

|
Scooped by
Gilbert C FAURE
July 3, 2024 2:03 PM
|
The New England Journal of Medicine has published a review of systemic amyloidosis. "Amyloidosis, a systemic disease that manifests in various ways, should be in the differential diagnosis of unexplained proteinuria, restrictive cardiomyopathy, peripheral and autonomic neuropathy, and hepatomegaly."AL (immunoglobulin light chain) amyloidosis is a rare disease that often results in progressive organ dysfunction, organ failure and eventual death.

|
Scooped by
Gilbert C FAURE
June 6, 2024 2:22 AM
|
Phagocytosis is the process by which myeloid phagocytes bind to and internalize potentially dangerous microorganisms1. During phagocytosis, innate immune receptors and associated signalling proteins are localized to the maturing phagosome compartment, forming an immune information processing hub brimming with microorganism-sensing features2–8. Here we developed proximity labelling of phagosomal contents (PhagoPL) to identify proteins localizing to phagosomes containing model yeast and bacteria. By comparing the protein composition of phagosomes containing evolutionarily and biochemically distinct microorganisms, we unexpectedly identified programmed death-ligand 1 (PD-L1) as a protein that specifically enriches in phagosomes containing yeast. We found that PD-L1 directly binds to yeast upon processing in phagosomes. By surface display library screening, we identified the ribosomal protein Rpl20b as a fungal protein ligand for PD-L1. Using an auxin-inducible depletion system, we found that detection of Rpl20b by macrophages cross-regulates production of distinct cytokines including interleukin-10 (IL-10) induced by the activation of other innate immune receptors. Thus, this study establishes PhagoPL as a useful approach to quantifying the collection of proteins enriched in phagosomes during host–microorganism interactions, exemplified by identifying PD-L1 as a receptor that binds to fungi. Proximity labelling of phagosomal contents is used to identify proteins that localize to phagosomes in host–microorganism interactions.

|
Scooped by
Gilbert C FAURE
May 28, 2024 11:18 AM
|
The majority of cancer patients receive radiotherapy. Wang et al. review preclinical and clinical data related to the importance of immune responses to rad

|
Scooped by
Gilbert C FAURE
May 13, 2024 4:39 AM
|

|
Scooped by
Gilbert C FAURE
May 7, 2024 11:37 AM
|
Leukocyte migration is a fundamental component of innate and adaptive immune responses as it governs the recruitment and localization of these motile cells, which is crucial for immune cell priming, effector functions, memory responses and immune regulation. This complex cellular trafficking system is controlled to a large extent via highly regulated production of secreted chemokines and the restricted expression of their membrane-tethered G-protein-coupled receptors. The activity of chemokines and their receptors is also regulated by a subfamily of molecules known as atypical chemokine receptors (ACKRs), which are chemokine receptor-like molecules that do not couple to the classical signalling pathways that promote cell migration in response to chemokine ligation. There has been a great deal of progress in understanding the biology of these receptors and their functions in the immune system in the past decade. Here, we describe the contribution of the various ACKRs to innate and adaptive immune responses, focussing specifically on recent progress. This includes recent findings that have defined the role for ACKRs in sculpting extracellular chemokine gradients, findings that broaden the spectrum of chemokine ligands recognized by these receptors, candidate new additions to ACKR family, and our increasing understanding of the role of these receptors in shaping the migration of innate and adaptive immune cells. This Review from Comerford and McColl discusses recent advances that have been made in understanding the biology of the atypical chemokine receptor (ACKR) family. The authors explain how these receptors interact with their ligands to shape immune responses and also highlight potential new additions to the ACKR family.
|

|
Scooped by
Gilbert C FAURE
September 18, 2024 7:30 AM
|
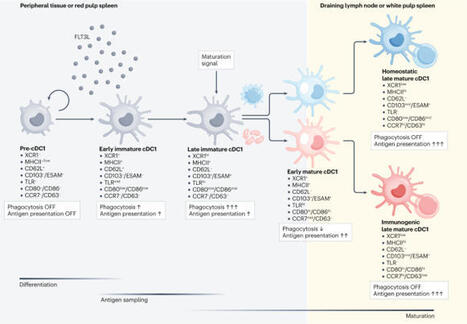
Dendritic cells (DCs) are crucial gatekeepers of the balance between immunity and tolerance. They exist in two functional states, immature or mature, that refer to an information-sensing versus an information-transmitting state, respectively. Historically, the term DC maturation was used to describe the acquisition of immunostimulatory capacity by DCs following their triggering by pathogens or tissue damage signals. As such, immature DCs were proposed to mediate tolerance, whereas mature DCs were associated with the induction of protective T cell immunity. Later studies have challenged this view and unequivocally demonstrated that two distinct modes of DC maturation exist, homeostatic and immunogenic DC maturation, each with a distinct functional outcome. Therefore, the mere expression of maturation markers cannot be used to predict immunogenicity. How DCs become activated in homeostatic conditions and maintain tolerance remains an area of intense debate. Several recent studies have shed light on the signals driving the homeostatic maturation programme, especially in the conventional type 1 DC (cDC1) compartment. Here, we highlight our growing understanding of homeostatic DC maturation and the relevance of this process for immune tolerance. Dendritic cells (DCs) act as gatekeepers between immunity and tolerance. Initially, it was postulated that mature DCs promote effector T cell responses and immature DCs promote tolerance. Recent studies have shown instead that two distinct modes of DC maturation exist — homeostatic and immunogenic. Here, Bosteels and Janssens discuss our current understanding of homeostatic DC maturation and how this contributes to immune tolerance, with a focus on the cDC1 compartment.

|
Scooped by
Gilbert C FAURE
September 10, 2024 1:29 PM
|
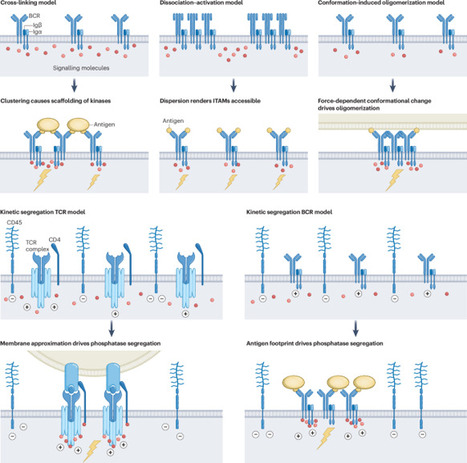
Antibodies are exceptionally versatile molecules with remarkable flexibility in their binding properties. Their natural targets range from small-molecule toxins, across viruses of different sizes, to bacteria and large multicellular parasites. The molecular determinants bound by antibodies include proteins, peptides, carbohydrates, nucleic acids, lipids and even synthetic molecules that have never existed in nature. Membrane-anchored antibodies also serve as receptors on the surface of the B cells that produce them. Despite recent structural insights, there is still no unifying molecular mechanism to explain how antibody targets (antigens) trigger the activation of these B-cell receptors (BCRs). After cognate antigen encounter, somatic hypermutation and class-switch recombination allow BCR affinity maturation and immunoglobulin class-specific responses, respectively. This raises the fundamental question of how one receptor activation mechanism can accommodate a plethora of variant receptors and ligands, and how it can ensure that individual B cells remain responsive to antigen after somatic hypermutation and class switching. There is still no definite answer. Here we give a brief historical account of the different models proposed to explain BCR triggering and discuss their merit in the context of the current knowledge of the structure of BCRs, their dynamic membrane distribution, and recent biochemical and cell biological insights. The mechanisms by which antigen triggers B-cell activation are incompletely understood. In this Review, Degn and Tolar discuss the different models of B-cell receptor triggering that have been proposed over the years in the light of recent insights.

|
Scooped by
Gilbert C FAURE
August 31, 2024 8:23 AM
|
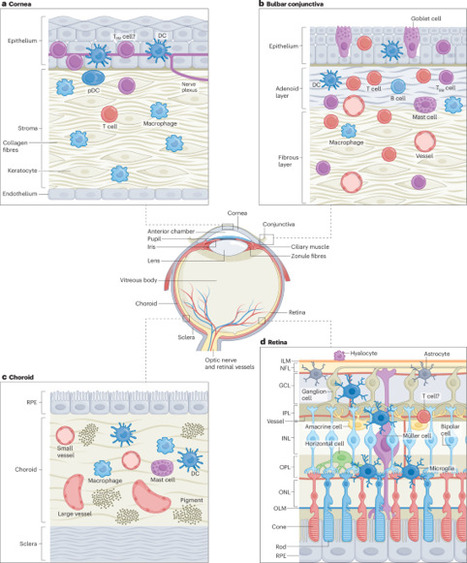
Balanced immune responses in the eyes are crucial to preserve vision. The ocular immune system has long been considered distinct, owing to the so-called ‘immune privilege’ of its component tissues. More recently, intravital imaging and transcriptomic techniques have reshaped scientific understanding of the ocular immune landscape, such as revealing the specialization of immune cell populations in the various tissues of the eye. As knowledge of the phenotypes of corneal and retinal immune cells has evolved, links to both the systemic immune system, and the central and peripheral nervous systems, have been identified. Using intravital imaging, T cells have recently been found to reside in, and actively patrol, the healthy human cornea. Disease-associated retinal microglia with links to retinal degeneration have also been identified. This Review provides an updated guide to the ocular immune system, highlighting current knowledge of the immune cells that are present in steady-state and specific diseased ocular tissues, as well as evidence for their relationship to systemic disease. In addition, we discuss emerging intravital imaging techniques that can be used to visualize immune cell morphology and dynamics in living human eyes and how these could be applied to advance understanding of the human immune system. This Review provides an overview of the immune system of the eye at steady state and in ocular disease, and it describes the links between ocular immunology and systemic disease. It highlights the intravital imaging techniques that have provided insights into immune cell morphology and dynamics in living human eyes.

|
Scooped by
Gilbert C FAURE
August 10, 2024 2:44 AM
|
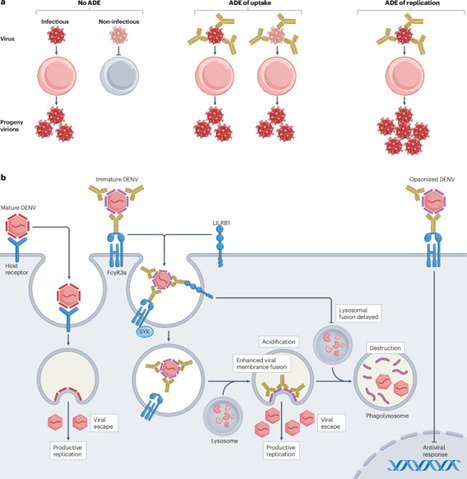
Antibody-dependent enhancement (ADE) of infectious disease is a phenomenon whereby host antibodies increase the severity of an infection. It is well established in viral infections but ADE also has an underappreciated role during bacterial, fungal and parasitic infections. ADE can occur during both primary infections and re-infections with the same or a related pathogen; therefore, understanding the underlying mechanisms of ADE is critical for understanding the pathogenesis and progression of many infectious diseases. Here, we review the four distinct mechanisms by which antibodies increase disease severity during an infection. We discuss the most established mechanistic explanation for ADE, where cross-reactive, disease-enhancing antibodies bound to pathogens interact with Fc receptors, thereby enhancing pathogen entry or replication, ultimately increasing the total pathogen load. Additionally, we explore how some pathogenic antibodies can shield bacteria from complement-dependent killing, thereby enhancing bacterial survival. We interrogate the molecular mechanisms by which antibodies can amplify inflammation to drive severe disease, even in the absence of increased pathogen replication. We also examine emerging roles for autoantibodies in enhancing the pathogenesis of infectious diseases. Finally, we discuss how we can leverage these insights to improve vaccine design and future treatments for infectious diseases. This Review discusses the different mechanisms of antibody-dependent enhancement (ADE) of infectious disease, including how antibodies can increase the pathogen load, protect bacteria from the immune system and amplify inflammation. The authors also highlight the role of autoantibodies and consider how a better understanding of ADE can be used to improve vaccines and treatments for infectious diseases.

|
Scooped by
Gilbert C FAURE
July 31, 2024 5:00 AM
|
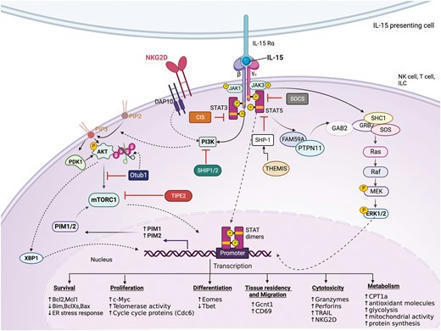
Summary. There is an intriguing dichotomy in the function of cytokine interleukin-15—at low levels, it is required for the homeostasis of the immune system

|
Scooped by
Gilbert C FAURE
July 11, 2024 4:11 AM
|

|
Scooped by
Gilbert C FAURE
July 10, 2024 10:04 AM
|
Written by Claire Kowalick A breakthrough for biomedical research promises new insight into immunotherapy development and disease modeling. Scientists at The University of Texas Health Science Center at San Antonio have created a humanized mouse model with a human immune system and a human-like gut microbiome that is capable of mounting specific antibody responses. The scientists […]

|
Scooped by
Gilbert C FAURE
July 8, 2024 11:22 AM
|
Natural killer (NK) cells are innate lymphoid cells (ILCs) that serve as a first line of defense against pathogens and tumors. Historically, their classification hinged on a limited array of surface protein markers. However, recent advancements in single-cell technologies, such assingle-cell RNA sequencing (scRNA-seq) and cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq), have unveiled a more complex and nuanced understanding of NK cells. This has led to variations in nomenclature and inconsistencies across scientific literature. In our study, we utilized these technologies to dissect the heterogeneity of NK cells in healthy human blood. We identified three prominent NK cell subsets: NK1, NK2 and NK3, further differentiated into six distinct subgroups. All subsets were present in all donors, irrespective of their human cytomegalovirus (HCMV) status. Our findings delineate the unique molecular characteristics, key transcription factors, principal biological functions, significant metabolic traits, and cytokine responses of each subgroup. These data also suggest two separate ontogenetic origins for NK cells, leading to divergent transcriptional trajectories. We explore these pathways in depth and propose a comprehensive, standardized nomenclature for NK cells. This standardized terminology aims at fostering clarity and consistency in future research, thereby improving cross-study comparisons.

|
Scooped by
Gilbert C FAURE
June 10, 2024 8:54 AM
|
Influenza virus infection is initiated by the attachment of the viral haemagglutinin (HA) protein to sialic acid receptors on the host cell surface. Most virus particles enter cells through clathrin-mediated endocytosis (CME). However, it is unclear how viral binding signals are transmitted through the plasma membrane triggering CME. Here we found that metabotropic glutamate receptor subtype 2 (mGluR2) and potassium calcium-activated channel subfamily M alpha 1 (KCa1.1) are involved in the initiation and completion of CME of influenza virus using an siRNA screen approach. Influenza virus HA directly interacted with mGluR2 and used it as an endocytic receptor to initiate CME. mGluR2 interacted and activated KCa1.1, leading to polymerization of F-actin, maturation of clathrin-coated pits and completion of the CME of influenza virus. Importantly, mGluR2-knockout mice were significantly more resistant to different influenza subtypes than the wild type. Therefore, blocking HA and mGluR2 interaction could be a promising host-directed antiviral strategy. Influenza virus uses metabotropic glutamate receptor 2 (mGluR2) as an endocytic receptor to enter cells through clathrin-mediated endocytosis.

|
Scooped by
Gilbert C FAURE
June 4, 2024 4:29 AM
|
Immune memory — comprising T cells, B cells and plasma cells and their secreted antibodies — is crucial for human survival. It enables the rapid and effective clearance of a pathogen after re-exposure, to minimize damage to the host. When antigen-experienced, memory T cells become activated, they proliferate and produce effector molecules at faster rates and in greater magnitudes than antigen-inexperienced, naive cells. Similarly, memory B cells become activated and differentiate into antibody-secreting cells more rapidly than naive B cells, and they undergo processes that increase their affinity for antigen. The ability of T cells and B cells to form memory cells after antigen exposure is the rationale behind vaccination. Understanding immune memory not only is crucial for the design of more-efficacious vaccines but also has important implications for immunotherapies in infectious disease and cancer. This ‘guide to’ article provides an overview of the current understanding of the phenotype, function, location, and pathways for the generation, maintenance and protective capacity of memory T cells and memory B cells. This Review provides a guide to the memory cells of the adaptive immune system, comprising memory T cells, memory B cells and plasma cells; it covers their formation, function, heterogeneity, localization, regulation and maintenance, and the crucial technological advances that allowed their discovery.

|
Scooped by
Gilbert C FAURE
May 27, 2024 7:08 AM
|
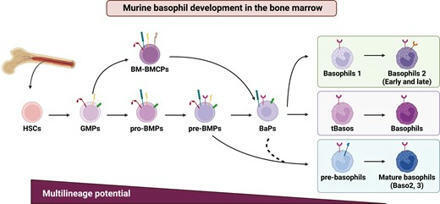
Summary. Basophils are the rarest leukocytes, but they have essential roles in protection against helminths, allergic disorders, autoimmune diseases, and s

|
Scooped by
Gilbert C FAURE
May 10, 2024 3:47 AM
|
CD4 T helper cells use the SNARE protein SYNTAXIN-11 to promote B cell differentiation, germinal center formation, and class switching by facilitating CD40
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...