The genetic mutation underlying a severe immune system deficiency can be corrected
by adenine base editing of human patient stem cells, restoring the ability of these
cells to develop into functional T cells in model systems, and paving the path for
future treatment of CD3δ SCID in patients.
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account

 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
Indiana University School of Medicine researchers have identified a new therapeutic target that could lead to more effective treatment of glaucoma.
BigField GEG Tech's insight:
Glaucoma is a neurodegenerative disease that causes vision loss and blindness due to a damaged optic nerve. Unfortunately, there is currently no cure. In a paper recently published in Communications Biology, researchers found that restoring mitochondrial homeostasis in diseased neurons can protect optic nerve cells from damage. The research team used induced pluripotent stem cells from glaucoma and non-glaucoma patients as well as clustered regularly spaced short palindromic repeats (CRISPRs) from human embryonic stem cells with glaucoma mutation. Using optic nerve stem cell differentiated retinal ganglion cells, electron microscopy and metabolic analysis, the researchers identified glaucomatous retinal ganglion cells with mitochondrial deficiency with a higher metabolic load on each mitochondrion, which leads to mitochondrial damage and degeneration. However, the process could be reversed by enhancing mitochondrial biogenesis with a pharmacological agent. The team showed that retinal ganglion cells are very efficient at degrading bad mitochondria, but at the same time produce more to maintain homeostasis.
Pancreatic cancer is an incurable form of cancer, and gene therapies are currently in clinical testing to treat this deadly disease. A comprehensive review of the gene and cell biotherapies in development to combat pancreatic cancer is published in the peer-reviewed journal Human Gene Therapy.
BigField GEG Tech's insight:
Pancreatic cancer is an incurable form of cancer and gene therapies are currently in clinical trials to treat this deadly disease. A comprehensive review of gene and cell biotherapies in development to combat pancreatic cancer is published in the peer-reviewed journal Human Gene Therapy. The article, "Pancreatic Cancer Cell and Gene Biotherapies: Past, Present and Future," by corresponding author Pierre Cordelier of the University of Toulouse, and co-authors describes ongoing gene therapy clinical trials. In addition to gene therapies, the authors discuss vaccines, chimeric antigen receptor (CAR) T-cell therapy, suicide genes and oncolytic viruses.
A new tool to predict the chances of successfully inserting a gene-edited sequence of DNA into the genome of a cell, using a technique known as prime editing, has been developed by researchers at the Wellcome Sanger Institute.
BigField GEG Tech's insight:
An evolution of the CRISPR-Cas9 gene editing technology, master editing has enormous potential to treat genetic diseases in humans. However, to date, the factors determining successful editing are not well understood. A new tool to predict the chances of successfully inserting a gene-edited DNA sequence into the genome of a cell has been developed by researchers. The study, published in Nature Biotechnology, evaluated thousands of different DNA sequences inserted into the genome using master editors. This data was then used to train a machine learning algorithm to help researchers design the best solution for a given genetic defect, promising to speed up efforts to introduce master editing into the clinic. Sequence length proved to be a key factor, as was the type of DNA repair mechanism involved. The next steps for the team will be to create models for all known human genetic diseases to better understand if and how they can be corrected using master editing.
Adding a molecular anchor to the key protein used to recognize cancer in cellular immunotherapies can make the treatments significantly more effective.
BigField GEG Tech's insight:
CAR T cells have shown some success in the clinic in treating certain cancers, such as relapsed leukemia. However, CAR T cells have not been successful in delivering solid tumors, in part because of problems with immune cell activation. Adding a molecular anchor to the key protein used to recognize cancer in cellular immunotherapies can make treatments much more effective. The researchers found that immune cells with the anchored protein increased cancer killing, regardless of their cell type or the type of cancer targeted. The concept of molecular anchoring is thus a new design for improving chimeric antigen receptor (CAR) based immunotherapies. Anchored CARs have increased survival in animal models of several tumor types, including lung, bone and brain cancers. CARs have shown promise in the clinic, but have not yet achieved widespread success in all tumor types. The findings were published in Nature Biotechnology.
Ablation of CaMKIIδ oxidation by CRISPR-Cas9 base editing as a therapy for cardiac disease - ScienceCRISPR-Cas9 gene editing is emerging as a prospective therapy for genomic mutations. However, current editing approaches are directed primarily toward relatively small cohorts of patients wit
BigField GEG Tech's insight:
A novel CRISPR-Cas9 approach that targets a damaging signaling pathway in the heart confers protection against ischemia and reperfusion injury, according to a study in mice. The findings suggest that gene editing could offer a permanent, advanced strategy for treating heart disease and even serve as an intervention to repair heart damage immediately after a heart attack. CRISPR-Cas9 gene editing has shown promise as a therapeutic approach for the treatment of rare inherited diseases. Chronic overactivation of Ca2+/calmodulin-dependent protein kinase IIδ (CaMKIIδ) is known to cause several heart diseases in humans and mice, including lesions. The researchers found that using CRISPR-Cas9 adenine base editing to eliminate oxidative activation sites of the CaMKIIδ gene in cardiomyocytes protected them from IR injury in mouse models. In addition, the researchers found that injecting gene-editing reagents into mice shortly after IR injury allowed the animals to recover cardiac function after severe damage.
The recent years have seen a wave of adoptive cell therapies (ACTs), a type of immunotherapy in which T cells (T cell transfer therapy) and other immune cells are obtained from patients, activated and multiplied outside the body, and infused in larger numbers back into the blood circulation to help fight cancers.
BigField GEG Tech's insight:
T-cell transfer therapies have not yet been successfully applied to solid tumors because T cells do not readily penetrate and persist in solid tumor masses for long periods of time, and because their activity is attenuated by an immunosuppressive tumor microenvironment. One way to overcome these limitations could be to couple T cell transfer therapies with cytokine therapy. However, a serious drawback of this approach is the significant side effects resulting from cytokines circulating freely in the body, leading to toxicity and potentially fatal inflammatory syndromes. Now, researchers have developed a nanotechnology-based solution to these problems. The method uses an unnatural sugar that is absorbed and embedded in the outer coating of T cells, which can then be used to anchor cytokines. The concentrated cytokines improve T-cell function locally without producing unwanted systemic side effects. In mice with melanoma, the approach also stimulated the host immune system against tumor cells, which inhibited tumor growth. As an adjunct to CAR-T cell therapy, it resulted in complete regression of lymphoma tumors at otherwise non-curative cell doses.
A Ludwig Cancer Research study has discovered that the immune system's surveillance of cancer can itself induce metabolic adaptations in the cells of early-stage tumors that simultaneously promote their growth and equip them to suppress lethal immune responses.
BigField GEG Tech's insight:
The immune system can induce metabolic adaptations in tumor cells at an early stage that promote their growth. Researchers identify three proteins that orchestrate this effect: IFNγ, STAT3 and c-Myc. The researchers show that IFNγ activates, in addition to the STAT1-mediated signaling pathway, the STAT3-mediated pathway. This pathway alters the genome expression patterns of the cancer cell by inducing epigenetic changes. It also hyperactivates c-Myc, which is overexpressed in many cancers. The researchers show that genes activated by c-Myc not only shape cancer metabolism, they also compromise T-cell infiltration into tumors and disable their attack on cancer cells. Thus, STAT1- and STAT3-mediated signaling pathways appear to synergize to confer on emerging tumors the ability to avoid immune clearance, resulting in the immunometabolic editing that helps fuel their evolution into full-blown malignancy. The researchers also used CRISPR to screen 2,078 metabolic enzymes in mouse tumors and identified 40 metabolic genes controlled by c-Myc that play an important role in helping cancer cells evade immune surveillance and attack. These enzymes are therefore prime candidates for drug targeting.
Huntington’s disease (HD) is a fatal, dominantly inherited neurodegenerative disorder caused by CAG trinucleotide expansion in exon 1 of the huntingtin (HTT) gene. Since the reduction of pathogenic mutant HTT messenger RNA is therapeutic, we developed a mutant allele-sensitive CAGEX RNA-targeting CRISPR–Cas13d system (Cas13d–CAGEX) that eliminates toxic CAGEX RNA in fibroblasts derived from patients with HD and induced pluripotent stem cell-derived neurons. We show that intrastriatal delivery of Cas13d–CAGEX via an adeno-associated viral vector selectively reduces mutant HTT mRNA and protein levels in the striatum of heterozygous zQ175 mice, a model of HD. This also led to improved motor coordination, attenuated striatal atrophy and reduction of mutant HTT protein aggregates. These phenotypic improvements lasted for at least eight months without adverse effects and with minimal off-target transcriptomic effects. Taken together, we demonstrate proof of principle of an RNA-targeting CRISPR–Cas13d system as a therapeutic approach for HD, a strategy with implications for the treatment of other dominantly inherited disorders. Leveraging RNA-targeting CRISPR–Cas13d technology, Morelli et al. engineered a novel therapeutic strategy that safely and effectively eliminates toxic expanded huntingtin RNA in multiple models of Huntington’s disease.
BigField GEG Tech's insight:
Huntington's disease (HD) is a neurological disorder that causes progressive loss of movement, coordination and cognitive function. It is caused by a mutation in a single gene called huntingtin or HTT. In a new study, published Dec. 12, 2022, in Nature Neuroscience, researchers describe the use of CRISPR/Cas13d RNA-targeting technology to develop a new therapeutic strategy that specifically removes the toxic RNA that causes HD. They used viral vehicles to deliver the therapy to neuronal cultures, which were developed from stem cells derived from patients with HD, and found that the approach not only targeted and destroyed mutant RNA molecules, but also eliminated the accumulation of toxic proteins. They also demonstrated that the expression of other human genes was generally not disrupted by the therapy.
In two separate studies, researchers demonstrate how synthetic biology can be used to tackle a difficult issue in cancer immunotherapy: the way immunotherapy-related approaches focused on short-term killing of tumor cells may fail to eradicate tumors because growth of tumors happens on longer timescales.
BigField GEG Tech's insight:
CAR T cell therapies are generally optimized for short-term cellular responses and may not allow for long-term systemic eradication of the tumor. To enable precise control of CAR T cell function over time, a first solution has been developed. Researchers have exploited recently developed synthetic Notch receptors to design enhanced CAR T cells with a second receptor. The second receptor can recognize a tumor antigen and then cause the T cell to release the cytokine interleukin-2, but when the CAR T cells are in direct contact with the tumor cells. In a mouse model, the approach enabled CAR T infiltration of solid pancreatic and melanoma tumors, resulting in substantial tumor eradication. In addition, a second solution was developed. Researchers developed a toolbox of 11 programmable synthetic transcription factors that could be activated on demand with timed administration of FDA-approved small-molecule inducers. Using these tools, the authors designed human immune cells that activate proliferation and antitumor activity on demand. The combination of the two technological advances presented will provide an unprecedented ability to precisely control the state of therapeutic cell populations not only at the time of injection but also as the immune response unfolds in the patient.
What drives tumor growth? Is it a few rogue cells imposing their will upon healthy tissue, or diseased tissue bringing out the worst in otherwise peaceable cells? Or is it a back-and-forth, a dialogue between the two?
BigField GEG Tech's insight:
Many cancer stem cells possess an RAS gene that, when mutated, allows tissue stem cells to ignore normal environmental signals and control tissue growth. To better understand the intricacies of this interaction, researchers turned their attention to squamous cell carcinoma. The team began by inducing mutant HRAS (the most common RAS family member in skin cancers) in individual skin stem cells and monitoring the interaction of the cancer stem cells with the surrounding tissue. This observation raised the possibility that many cancer mutations do not fix the course of a disease but lock it in place, affirming a malignant progression already determined by aberrant crosstalk between a cancer stem cell and its microenvironment. By further studying how the cancer stem cell changed in the face of this new self-imposed malignant tumor microenvironment, the team realized that invasive cancer stem cells unexpectedly began expressing the leptin receptor, Lepr. The researchers used CRISPR technology to show that Lepr and leptin receptor signaling were critical for progression from benign to malignant. The team is now investigating ways to block leptin receptors in tumors because doing so could throw a molecular monkey wrench into the vicious loop and derail the cancer.
Researchers at VCU Massey Cancer Center have set their sights on a new therapeutic target for an aggressive form of breast cancer with limited treatment options.
BigField GEG Tech's insight:
Breast cancer is the second most common cancer in American women, and triple-negative breast cancer (TNBC) is a more aggressive and deadly form of the disease that accounts for 10-15% of all breast tumors. Using a comprehensive, state-of-the-art genomic screening method known as CRISPR/CAS9 screening, scientists were able to identify a specific enzyme called UBA1 that proved to be an ideal therapeutic target. Using a new UBA inhibitor drug called TAK-243, they blocked the cellular function of UBA1 and effectively killed cancer cells in patient-derived breast tumors in mice. Previous research has shown that UBA1 inhibitors can have a positive impact on hematological cancers such as acute myeloid leukemia and chronic myeloid leukemia. This study, recently published in PNAS Nexus, is the first to suggest that UBA1 inhibitors may be effective in TNBC. TAK-243 was recently tested in early phase trials, paving the way for potential testing in TNBC patients. The researchers also determined that the c-MYC gene can be harnessed to cooperate with TAK-243 to initiate a cellular stress response and improve the drug's ability to combat TNBC. This supports the idea that TAK-243 may be effective in TNBC with high c-MYC expression, where c-MYC may serve as a biomarker for drug response.
A new approach to cancer immunotherapy that uses one type of immune cell to kill another-;rather than directly attacking the cancer-;provokes a robust anti-tumor immune response that shrinks ovarian, lung, and pancreatic tumors in preclinical disease models, according to researchers at the Icahn School of Medicine at Mount Sinai in New York.
BigField GEG Tech's insight:
CAR T cells currently in clinical use are designed to recognize cancer cells directly and have successfully treated several blood cancers. But there have been challenges that prevent their effective use in many solid tumors. Most solid tumors are heavily infiltrated by a type of immune cell called macrophages. Macrophages help tumors grow by blocking the entry of T cells into the tumor tissue, which prevents CAR T cells and the patient's own T cells from destroying the cancer cells. To address this immune suppression at the source, the researchers engineered T cells to make a chimeric antigen receptor that recognizes a molecule on the surface of macrophages. When these CAR T cells encountered a tumor macrophage, the CAR T cell became activated and killed the tumor macrophage. Treating mice with ovarian, lung and pancreatic tumors with these macrophage-targeting CAR T cells reduced the number of tumor macrophages, shrank the tumors and prolonged their survival. The destruction of tumor macrophages allowed the mice's own T cells to access and kill the cancer cells. The researchers further demonstrated that this anti-tumor immunity was induced by the release of the cytokine interferon-gamma by CAR T cells.
|
A new approach that delivers a "one-two punch" to help T cells attack solid tumors is the focus of a preclinical study by researchers from the Perelman School of Medicine at the University of Pennsylvania.
BigField GEG Tech's insight:
One of the challenges of CAR T cell therapy in solid tumors is a phenomenon known as T cell exhaustion. Previous studies have alluded to the inflammatory regulator Regnase-1 as a potential target to indirectly overcome the effects of T-cell exhaustion, as it can cause hyperinflammation when disrupted in T cells, reviving them to produce an antitumor response. The research team hypothesized that targeting the related but independent Roquin-1 regulator at the same time could boost responses further. The team used CRISPR-Cas9 gene editing to knock out Regnase-1 and Roquin-1 individually and together in healthy donor T cells with two different immune receptors that are currently being studied in Phase I clinical trials: the mesothelin-targeting M5 CAR (mesoCAR) and the NY-ESO-1-targeting 8F TCR (NYESO TCR). Following CRISPR editing, the T cells were expanded and infused into solid tumor mouse models, where the researchers observed that the double knockout resulted in at least a 10-fold increase in modified T cells compared to knocking down Regnase-1 alone, as well as increased anti-tumor immune activity and longevity of modified T cells. In some mice, this also led to an overproduction of lymphocytes, causing toxicity.
Range of DNA repair in response to double-strand breaks induced in human preimplantation embryos remains uncertain due to the complexity of analyzing single- or few-cell samples. Sequencing of such minute DNA input requires a whole genome amplification that can introduce artifacts, including coverage nonuniformity, amplification biases, and allelic dropouts at the target site. We show here that, on average, 26.6% of preexisting heterozygous loci in control single blastomere samples appear as homozygous after whole genome amplification indicative of allelic dropouts. To overcome these limitations, we validate on-target modifications seen in gene edited human embryos in embryonic stem cells. We show that, in addition to frequent indel mutations, biallelic double-strand breaks can also produce large deletions at the target site. Moreover, some embryonic stem cells show copy-neutral loss of heterozygosity at the cleavage site which is likely caused by interallelic gene conversion. However, the frequency of loss of heterozygosity in embryonic stem cells is lower than in blastomeres, suggesting that allelic dropouts is a common whole genome amplification outcome limiting genotyping accuracy in human preimplantation embryos. DNA repair in response to DSBs in the preimplantation embryo is hard to analyze. Here the authors show that over 25% of pre-existing heterozygous loci in control single blastomere samples appeared as homozygous after whole genome amplification, therefore, they validated gene editing seen in human embryos in ESCs.
BigField GEG Tech's insight:
Although gene editing technologies hold promise for preventing and treating debilitating inherited diseases, a new study reveals limitations that must be overcome before gene editing to establish a pregnancy can be considered safe or effective. The study, published recently in the journal Nature Communications, involved sequencing the genomes of early human embryos that had undergone genome editing using the CRISPR gene editing tool. The work calls into question the accuracy of a DNA reading procedure that relies on amplifying a small amount of DNA for genetic testing. This scientific method commonly used to analyze a tiny amount of DNA in early human embryos fails to accurately reflect genetic changes. In addition, the study also reveals that gene editing to correct disease-causing mutations in early human embryos can also result in unintended and potentially harmful changes to the genome. The findings raise a new scientific basis for caution for any scientist who might be about to use genetically modified embryos to establish pregnancies.
The lack of registered drugs for nonalcoholic fatty liver disease (NAFLD) is partly due to the paucity of human-relevant models for target discovery and compound screening. Here we use human fetal hepatocyte organoids to model the first stage of NAFLD, steatosis, representing three different triggers: free fatty acid loading, interindividual genetic variability (PNPLA3 I148M) and monogenic lipid disorders (APOB and MTTP mutations). Screening of drug candidates revealed compounds effective at resolving steatosis. Mechanistic evaluation of effective drugs uncovered repression of de novo lipogenesis as the convergent molecular pathway. We present FatTracer, a CRISPR screening platform to identify steatosis modulators and putative targets using APOB−/− and MTTP−/− organoids. From a screen targeting 35 genes implicated in lipid metabolism and/or NAFLD risk, FADS2 (fatty acid desaturase 2) emerged as an important determinant of hepatic steatosis. Enhancement of FADS2 expression increases polyunsaturated fatty acid abundancy which, in turn, reduces de novo lipogenesis. These organoid models facilitate study of steatosis etiology and drug targets. Organoid models of early liver disease aid target discovery and drug screening.
BigField GEG Tech's insight:
The accumulation of fat in the liver is an increasingly common disease worldwide, with more than a quarter of the world's population affected. Various causes can lead to the development of fatty liver, with diet and lifestyle being the most common contributors. There is currently no treatment for fatty liver that can stop or reverse the disease. Therefore, researchers have established new human organoid models of fatty liver. They used these models to shed light on drug responses and established a CRISPR screening platform, called FatTracer, to identify new disease mediators and potential therapeutic targets. After screening 35 candidates, a critical new role for the FADS2 (fatty acid desaturase 2) gene in fatty liver disease was discovered. Disruption of FADS2 made the organoids much fatter. Upon overexpression of FADS2, the hepatic steatosis that the organoids once displayed was severely reduced. These models will therefore help test and develop new drugs to treat hepatic steatosis and better understand the biology of the disease. The results of the study will be published in Nature Biotechnology.
Understanding moral acceptability and willingness to use is crucial for informing policy
BigField GEG Tech's insight:
Preimplantation genetic testing has become standard care for parents at risk of having children with chromosomal and single gene disorders. Embryos created via in vitro fertilization (IVF) can be genetically screened for abnormalities prior to implantation, thus minimizing the risk of inherited genetic disease. However, most human traits are highly polygenic. Preimplantation genetic testing for polygenic risk (PGT-P) is an emerging technology that can screen the entire genome of an embryo and uses polygenic indices to predict the likelihood of a particular polygenic phenotype occurring if used in IVF. Targeted outcomes range from risk of cancer and other diseases to a child's potential educational attainment. Although PGT-P is available in IVF clinics around the world, it remains unregulated in the United States and has received far less public attention and policy discussion than other technologies that seek to exert control over genetic traits, such as germline gene editing via CRISPR. To better understand the public's views toward PGT-P, researchers conducted a prerecorded national survey experiment on attitudes toward PGT-P. The authors found that moral acceptability and willingness to use PGT-P were higher than those for germline gene editing.
Dual-action cell therapy engineered to eliminate established tumors and train the immune system to eradicate primary tumor and prevent cancer’s recurrence.
BigField GEG Tech's insight:
Cancer vaccines are an active area of research for many laboratories. However, one team of researchers has taken an approach that is distinct from other labs. Instead of using inactivated tumor cells, the researchers are reusing live tumor cells that possess the characteristic of traveling long distances to return to the site of their tumor counterparts. Taking advantage of this unique property, the research team engineered live tumor cells using the CRISPR-Cas9 gene-editing tool and reused them to release tumor cell killing agents. In addition, the modified tumor cells were engineered to express factors that would make them easy for the immune system to spot, tag, and remember, preparing the immune system for a long-term antitumor response. The researchers tested their CRISPR-enhanced, reverse-engineered therapeutic tumor cells (ThTCs) in different strains of mice, including one that carried human-derived bone marrow, liver and thymus cells, mimicking the human immune microenvironment. The researchers also built a two-layer safety switch in the cancer cell that, when activated, eradicates ThTCs if necessary. The promising results are published in Science Translational Medicine.
With a slew of tools to trick out immune cells, researchers are expanding the repertoire of CAR-T therapies.
BigField GEG Tech's insight:
Crystal Mackall remembers her scepticism the first time she heard a talk about a way to engineer T cells to recognize and kill cancer. Sitting in the audience at a 1996 meeting in Germany, the paediatric oncologist turned to the person next to her and said: “No way. That’s too crazy.” Today, things are different. “I’ve been humbled,” says Mackall, who now works at Stanford University in California developing such cells to treat brain tumours.
BigField GEG Tech's insight:
ALLO-715 is a first-in-class, allogeneic, anti-BCMA CAR T cell therapy engineered to abrogate graft-versus-host disease and minimize CAR T rejection. The scientists evaluated escalating doses of ALLO-715 after lymphodepletion with an anti-CD52 antibody (ALLO-647)-containing regimen in 43 patients with relapsed/refractory multiple myeloma as part A of the ongoing first-in-human phase 1 UNIVERSAL trial.
Researchers at UCSF have developed a novel, potentially life-saving approach that may prevent antibodies from triggering immune rejection of engineered therapeutic and transplant cells.
BigField GEG Tech's insight:
Until recently, most CAR-T therapies were made from the patient's own cells, but the long-term commercial viability of cell therapies of all types will rely on "allogeneic" cells, therapeutic cells mass-produced and grown from a source outside the patient. However, the recipient's immune system is likely to treat all outside cells as foreign and reject them. Immune rejection of therapeutic cells, such as CAR-T cells, mediated by antibodies, as opposed to chemical aggression initiated by them, has proven to be particularly challenging to resolve. This is a factor that hinders the development of these treatments. Researchers have therefore developed a method to catch antibodies before they bind to cells, preventing the activation of the immune response. The researchers genetically engineered three types of cells : insulin-producing islet cells, thyroid cells and CAR-T cells, so that each type makes and displays a large number of a protein called CD64 on their surface. On these engineered cells, CD64, which tightly binds the antibodies responsible for this type of immune rejection, acted as a kind of decoy, capturing the antibodies and binding them to the engineered cell, so that they would not activate the immune cells. The new strategy is described in Nature Biotechnology.
Bacterial abortive-infection systems limit the spread of foreign invaders by shutting down or killing infected cells before the invaders can replicate1,2. Several RNA-targeting CRISPR–Cas systems (that is, types III and VI) cause abortive-infection phenotypes by activating indiscriminate nucleases3–5. However, a CRISPR-mediated abortive mechanism that leverages indiscriminate DNase activity of an RNA-guided single-effector nuclease has yet to be observed. Here we report that RNA targeting by the type V single-effector nuclease Cas12a2 drives abortive infection through non-specific cleavage of double-stranded DNA (dsDNA). After recognizing an RNA target with an activating protospacer-flanking sequence, Cas12a2 efficiently degrades single-stranded RNA (ssRNA), single-stranded DNA (ssDNA) and dsDNA. Within cells, the activation of Cas12a2 induces an SOS DNA-damage response and impairs growth, preventing the dissemination of the invader. Finally, we harnessed the collateral activity of Cas12a2 for direct RNA detection, demonstrating that Cas12a2 can be repurposed as an RNA-guided RNA-targeting tool. These findings expand the known defensive abilities of CRISPR–Cas systems and create additional opportunities for CRISPR technologies. RNA targeting by the Sulfuricurvum type V single-effector nuclease SuCas12a2 drives abortive infection through non-specific cleavage of double-stranded DNA—after recognition of an RNA target through an activating protospacer-flanking sequence, SuCas12a2 efficiently degrades ssRNA, ssDNA and dsDNA.
BigField GEG Tech's insight:
Scientists at the Helmholtz Institute Würzburg in Germany, and Benson Hill, Inc. (Missouri) and Utah State University in the U.S., have found a nuclease, which they dubbed Cas12a2, that represents an entirely new type of CRISPR immune defense. Unlike any other previously known nuclease of the CRISPR-Cas immune system, the source of ‘gene scissors,’ Cas12a2 destroys DNA to shut down an infected cell. The findings could lead to new CRISPR technologies for molecular biology diagnostics, among other applications.
Precision-controlled CAR-T-cell immunotherapies could be used to tackle a range of tumour types.
BigField GEG Tech's insight:
Researchers have bolstered the power of chimeric antigen receptor (CAR)-T cancer therapies, which use genetically altered T cells to seek out tumours and mark them for destruction. Now scientists have further engineered the cells to contain switches that allow control over when and where the cells are active. This helps them to infiltrate tumours and dodge immune-suppressing defences.
Last week, PACT Pharma shared results from the first clinical trial using CRISPR to direct patients’ immune cells to treat solid tumours. The findings, which were published in an unedited manuscript in Nature, provide early proof-of-concept that patient immune cells can be reprogrammed to attack their own cancer. The results were als
BigField GEG Tech's insight:
In most cases, the number of naturally occurring cancer-targeting T cells will be far too low to trigger an immune response capable of eradicating a patient's tumor(s). PACT Pharma is a privately held biopharmaceutical company developing personalized, neo-antigen-specific T-cell receptor (TCR)-T therapies to treat a range of solid tumors. Patient T cells are isolated and CRISPR-modified by electroporation with Cas9 protein, guide RNAs to knock out endogenous TCR genes (TCRα ( TRAC) and TCRβ ( TRBC)) and an HR template plasmid encoding the transgenic neoTCR. The results of a phase 1 clinical trial that were published in Nature. The researchers report that CRISPR-modified T cells were preferentially directed to the tumor and could be recovered from post-infusion biopsies in all patients for whom biopsies were available. They also note that CRISPR-edited T cells frequently accounted for the top 2-20% of immune cells in the tumor, and a reduction in tumor size was observed in some lesions in a single lung cancer patient.
Pierre-Luc Jellimann 's curator insight,
December 1, 2022 3:01 AM
Nouvelle article montrant l'efficaicté de CRISPR dans le traitement des tumeurs solides (first clinical trial using CRISPR to direct patients’ immune cells to treat solid tumours)
Researchers at Great Ormond Street Hospital for Children (GOSH) and UCL Great Ormond Street Institute of Child Health (UCL GOS ICH) have used CRISPR/Cas9 technology to engineer donor T cells to try to treat seriously ill children with resistant leukaemia, who had otherwise exhausted all available therapies.
BigField GEG Tech's insight:
Researchers have used CRISPR/Cas9 technology to engineer donor T cells to treat critically ill children with resistant leukemia who had otherwise exhausted all available therapies. The Phase I trial, published in Science Translational Medicine , is the first use of CRISPR-edited "universal" cells in humans and represents a significant advance in the use of genetically engineered cells for cancer treatment. In the trial, the research team constructed and applied a new generation of genome-edited "universal" T cells, which builds on previous work that used older, less precise technology. The T cells were modified using CRISPR. In addition, the piece of genetic code allows the T cells to express a CAR receptor that can recognize a marker on the surface of cancerous B cells and then destroy them. The T cells were then genetically modified with CRISPR so that they could be used "off the shelf" without the need for a donor match. In specialist clean rooms, researchers manufactured their banks of donor CAR T-cells using a single disabled virus to transfer both the CAR and a CRISPR guidance system, and then applied cutting-edge mRNA technology to activate the gene editing steps.
Pierre-Luc Jellimann 's curator insight,
November 17, 2022 3:03 AM
Researchers have used CRISPR/Cas9 technology to engineer donor T cells to treat critically ill children with resistant leukemia who had otherwise exhausted all available therapies. The Phase I trial, published in Science Translational Medicine , is the first use of CRISPR-edited "universal" cells in humans and represents a significant advance in the use of genetically engineered cells for cancer treatment. In the trial, the research team constructed and applied a new generation of genome-edited "universal" T cells, which builds on previous work that used older, less precise technology. The T cells were modified using CRISPR. In addition, the piece of genetic code allows the T cells to express a CAR receptor that can recognize a marker on the surface of cancerous B cells and then destroy them. The T cells were then genetically modified with CRISPR so that they could be used "off the shelf" without the need for a donor match. In specialist clean rooms, researchers manufactured their banks of donor CAR T-cells using a single disabled virus to transfer both the CAR and a CRISPR guidance system, and then applied cutting-edge mRNA technology to activate the gene editing steps. 
Singh's curator insight,
November 17, 2022 5:07 AM
Une thérapie génique et universelle. La technologie CRISPR/Cas9 est un espoir dans la thérapie de la leucémie résistante. Une attention toute particulière doit toutefois se porter sur l'insertion génétique qui doit être finement régulée afin d'éviter les effets délétères. 
Gilbert C FAURE's comment,
November 17, 2022 6:23 AM
allez voir sur https://www.scoop.it/topic/immunology-and-biotherapies quelques surprises déjà New study finds that CRISPR/Cas9 leads to unexpected genomic changes
|












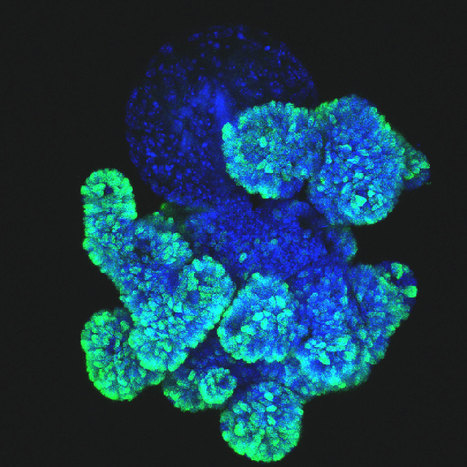







The rare and fatal genetic disease CD3 delta severe combined immunodeficiency, also known as CD3 delta SCID, is caused by a mutation in the CD3D gene, which prevents the production of the CD3 delta protein needed for normal T cell development from blood stem cells. Currently, bone marrow transplantation is the only treatment available, but the procedure carries significant risks. In a study published in Cell, researchers showed that a new genome editing technique called base editing can correct the mutation that causes CD3 delta SCID in blood stem cells and restore their ability to produce T cells. The basic editor corrected an average of nearly 71 percent of the patient's stem cells in three experiments. The researchers then tested whether the corrected cells could give rise to T cells. When the corrected blood stem cells were introduced into artificial thymic organoids, they produced fully functional and mature T cells. The corrected cells remained four months after transplantation, indicating that the basic editing had corrected the mutation in the true self-renewing blood stem cells. The results suggest that the corrected blood stem cells could persist over the long term and produce the T cells that patients would need to lead healthy lives.