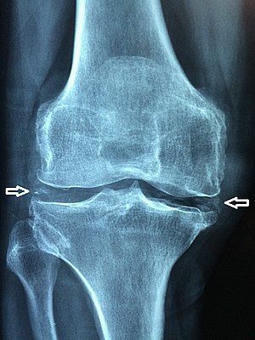
Abstract Objective To estimate the prevalence and distribution of asymptomatic monosodium urate monohydrate (MSU) crystal deposition in sons of patients with gout. Methods Patients with gout were mailed an explanatory letter with an enclosed postage‐paid study packet to mail to their son(s) age ≥20 years old. Sons interested in participating returned a reply form and underwent telephone screening. Subsequently, they attended a study visit at which blood and urine samples were obtained and musculoskeletal ultrasonography was performed, with the sonographer blinded with regard to the subject's serum urate level. Images were assessed for double contour sign, intraarticular or intratendinous aggregates/tophi, effusion, and power Doppler signal. Logistic regression was used to examine associations. Adjusted odds ratios (ORadj) and 95% confidence intervals (95% CIs) were calculated. Results One hundred thirty‐one sons (mean age 43.8 years, mean body mass index 27.1 kg/m2) completed assessments. The serum urate level was ≥6 mg/dl in 64.1%, and 29.8% had either a double contour sign or intraarticular aggregates/tophi in ≥1 joint. All participants with MSU deposition had involvement of 1 or both first metatarsophalangeal joints. Intratendinous aggregates were present in 21.4% and were associated with intraarticular MSU crystal deposits (ORadj 2.96 [95% CI 1.17–7.49]). No participant with a serum urate level of ≤5 mg/dl had MSU crystal deposition seen on ultrasonography, and 24.2% of those with serum urate levels between 5 and 6 mg/dl had ultrasonographic MSU deposition. MSU crystal deposition was associated with increasing serum urate levels (ORadj 1.61 [95% CI 1.10–2.36] for each increase of 1 mg/dl). Conclusion Asymptomatic sons of patients with gout frequently have hyperuricemia and MSU crystal deposits. In this study MSU crystal deposits were present in participants with serum urate levels of ≥5 mg/dl. Evaluation of subjects without a family history of gout is needed to determine whether the threshold for MSU crystal deposition is also lower in the general population. Gout is the most common form of inflammatory arthritis and results from persistent hyperuricemia that causes intra‐ and periarticular monosodium urate monohydrate (MSU) crystal deposition. It has multiple risk factors including genetic factors that act by modulating renal uric acid excretion 1. The heritability of serum urate and urinary uric acid excretion is estimated to be ~60% and 60–87%, respectively 2, while the heritability of gout is lower at 17.0% and 35.1% in Taiwanese women and men, respectively 3, and was estimated to range between 0% and 58% in a study from the US 1. As 14.5–25% of people with high serum urate levels have asymptomatic MSU crystal deposition 4-6, studies that use a symptomatic disease phenotype may underestimate the heritability of MSU crystal deposition. The prevalence of asymptomatic MSU crystal deposition in people at high genetic risk of gout, e.g., those with a parent who has gout, is not known. It has implications for screening and primary prevention of symptomatic gout and contrasts with rheumatoid arthritis, in which familial risk and prevalence of autoantibodies in first‐degree relatives are well understood. Thus, we undertook the present study to 1) examine the prevalence and distribution of asymptomatic MSU crystal deposition among sons of people with gout; 2) examine the association between serum urate, age, and asymptomatic MSU crystal deposition; and 3) explore whether parental age at gout onset is associated with asymptomatic MSU crystal deposition in sons. Patients and Methods Study design, subject recruitment, and ethics approval. This community‐based cross‐sectional study was approved by the Nottingham NHS Research Ethics Committee 2 (Rec ref: 15/EM/0316). People with self‐reported physician‐diagnosed gout who had participated in previous surveys at Academic Rheumatology, University of Nottingham and consented to future research contact were mailed a letter informing them of the present study and were asked to mail an enclosed study packet to their sons age ≥20 years. Sons of patients with primary gout who attended the rheumatology clinic at the Nottingham NHS Treatment Centre were approached in a similar manner, and the study was advertised on Facebook and once in a local newspaper. These advertisements were targeted at sons living in and around Nottingham who have a parent with gout. Sons who returned a reply form or contacted us in response to the advertisements underwent a telephone screening questionnaire to exclude those with gout 7. The screening questionnaire included questions that form part of the 8‐point chronic gout diagnosis (CGD) scale 7. The CGD scale includes current or past history of attack of acute arthritis, monoarthritis or oligoarthritis, rapid progression of pain and swelling (<24 hours), podagra, erythema, unilateral tarsitis, tophi, and hyperuricemia 7. As serum urate was not measured at the screening visit in this study, a history of hyperuricemia was substituted. Participants scoring ≤3 on the CGD were invited for the study visit. Study visit. Participants attended a study visit at which data on demographic characteristics, lifestyle factors, comorbidities, and drug prescriptions were collected. Targeted musculoskeletal assessment was performed, height, weight, and blood pressure were measured, and random blood and second‐void early‐morning urine samples were collected. Serum urate and creatinine and urinary uric acid and creatinine were measured at the clinical pathology laboratories of Nottingham University Hospitals NHS Trust. Fractional excretion of uric acid (FEUA) was calculated as ([urinary uric acid × serum creatinine]/[serum urate × urinary creatinine]) × 100% 8. Ultrasonography was performed by a rheumatologist with 5 years of ultrasonography experience (AA), who was blinded with regard to the subject's serum urate level. The ultrasonographic examination involved assessment of both first metatarsophalangeal (MTP) joints, talar domes, femoral condyles, second metacarpophalangeal joints, wrist triangular fibrocartilages, and patellar and triceps tendon insertions 9. These joints and tendons were chosen as they have best sensitivity and specificity for differentiating subjects with gout from those with other arthropathies 9. Ultrasound images were scored for double contour sign, intraarticular or intratendinous tophi/aggregates, and hyperechoic deposits (present or absent, as defined by the Outcome Measures in Rheumatology group 10). Joint effusion and power Doppler signal were graded on a 0–3 scale. All ultrasonographic assessments were performed using a Toshiba Aplio machine (8–14 MHz). Images with inconclusive readings were reviewed by a second ultrasonographer with >15 years of ultrasonography experience (PC), also under blinded conditions with regard to the subject's serum urate level. For the purpose of this study, MSU crystal deposits were defined as present if there was an intraarticular double contour sign or tophi/aggregates. Hyperechoic deposits alone were not sufficient to define MSU crystal deposits. When available, data on sex of the parent with gout and age at onset of gout were extracted from databases at Academic Rheumatology, University of Nottingham. Statistical analysis. The mean ± SD and the number (%) were used to describe continuous and categorical data, respectively. Independent‐sample t‐tests and chi‐square tests were used for univariate analysis; the Kruskal‐Wallis test was used if the data were nonparametric. Logistic regression was used to examine the association between intraarticular MSU crystal deposition at any joint in an individual and 1) serum urate level, 2) FEUA, 3) age, and 4) intratendinous aggregates/tophi at any tendon. The associations were adjusted for age where required, body mass index (kg/m2), current purine‐rich alcohol consumption (yes/no), hypertension (yes/no), hyperlipidemia (yes/no), diabetes (yes/no), estimated glomerular filtration rate (ml/minute), and father with gout (yes/no). Adjusted odds ratios (ORadj) and 95% confidence intervals (95% CIs) were calculated. The individual was the unit of analysis. Statistical calculations were performed using Stata version 15. P values less than 0.05 were considered significant. Results One hundred thirty‐four participants were recruited into the study: 125 via postal survey (1,435 study packets sent, 249 replies received), 6 from among sons of gout patients attending Nottingham University Hospitals NHS Trust, and 3 from advertisements. The 3 individuals recruited from advertisements did not present for ultrasonographic assessment and were excluded from further analysis. The serum urate level was ≥6 mg/dl in 64.1% of the subjects and ≥7 mg/dl in 29.0%. Demographic characteristics and comorbidities of the 131 participants are summarized in Table 1. The mean ± SD FEUA was 5.33 ± 1.87%, and FEUA was low (defined as ≤6.6%) in 78.6% of the subjects with a serum urate level of ≥6 mg/dl. Total Asymptomatic MSU crystal deposition Present (n = 39) Absent (n = 92) Age, mean ± SD years 43.80 ± 11.20 44.20 ± 8.91 43.63 ± 12.08 Age 20–29 years, no. (%) 20 (15.3) 3 17 Age 30–39 years, no. (%) 27 (20.6) 10 17 Age 40–49 years, no. (%) 40 (30.5) 15 25 Age 50–59 years, no. (%) 36 (27.5) 10 26 Age 60–69 years, no. (%) 8 (6.1) 1 7 Body mass index, mean ± SD kg/m2 27.10 ± 4.75 27.65 ± 3.99 26.85 ± 5.04 Current purine‐rich alcohol consumption, no. (%) 97 (74.1) 31 (79.5) 66 (71.7) Weekly purine‐rich alcohol intake, median (IQR) units 10 (5–20) 10 (5–20) 10 (4–20) Hypertension, no. (%) 12 (9.2)b 3 (7.7) 9 (9.8) Hyperlipidemia, no. (%) 10 (7.6)c 3 (7.7) 7 (7.6) Diabetes, no. (%) 2 (1.5)d 0 2 (2.2) eGFR, mean ± SD ml/minute 85.23 ± 7.19 85.21 ± 7.71 85.24 ± 7.00 Serum urate, mean ± SD mg/dl 6.41 ± 1.13 6.79 ± 0.96e 6.25 ± 1.16 Serum urate <5 mg/dl, no. (%) 14 (10.5) 0 14 Serum urate ≥5 and <6 mg/dl, no. (%) 33 (26.9) 8 25 Serum urate ≥6 and <7 mg/dl, no. (%) 46 (34.3) 18 28 Serum urate ≥7 and <8 mg/dl, no. (%) 27 (20.2) 8 19 Serum urate ≥8 and <9 mg/dl, no. (%) 9 (6.7) 4 5 Serum urate ≥9 mg/dl, no. (%) 2 (1.5) 1 1 FEUA, mean ± SD % 5.3 ± 1.9 5.3 ± 1.7 5.3 ± 1.9 Father with gout, no. (%) 111 (84.7) 33 (84.6) 78 (84.8) a All participants with asymptomatic monosodium urate monohydrate (MSU) crystal deposition had first metatarsophalangeal joint involvement. IQR = interquartile range; eGFR = estimated glomerular filtration rate; FEUA = fractional excretion of uric acid. b Eleven participants were prescribed antihypertensive drugs; 1 received bendroflumethiazide. c Six participants were prescribed statins. d Both participants were prescribed oral hypoglycemic drugs. e P = 0.01 versus participants without asymptomatic MSU crystal deposition. MSU crystal deposition was found in 29.8% of the subjects, with involvement of the first MTP joint (Figure 1) observed in all subjects in whom asymptomatic MSU crystal deposition was present. MSU crystal deposition was not found in any participant with a serum urate level of ≤5 mg/dl. Among the 262 first MTP joints examined, intraarticular aggregates were numerically more common than double contour sign (Table 2). Only 1 participant had a double contour sign at the ankle, and MSU crystal deposits were not present at the other joints examined. MSU crystal deposition at the first MTP joint was associated with grade ≥2 effusion at the same joint (ORadj 9.44 [95% CI 3.62–24.63], ORadj 5.44 [95% CI 1.57–18.82] for the right and left sides, respectively). The power Doppler signal was grade ≥2 in only 1 first MTP joint. Asymptomatic MSU deposition in first MTP joints Present (n = 49) Absent (n = 213) First MTP joints Double contour sign 13 (5.0) – – Tophi 27 (10.3) – – Double contour sign and tophi 9 (3.4) – – Grade ≥2 effusion 56 (21.4) 26 30 Patellar tendon Hyperechoic deposits 19 (7.3) 11 8 Unilateral 13 7 6 Bilateral 3 2 1 Triceps tendon Hyperechoic deposits 15 (5.7) 8 7 Unilateral 13 6 7 Bilateral 1 1 0 a One participant had a double contour sign at 1 ankle; triangular fibrocartilage involvement was not observed in any participant. Values are the number (%) of joints/tendons. MSU = monosodium urate monohydrate; MTP = metatarsophalangeal. Hyperechoic aggregates were present in at least 1 tendon in 28 participants (21.4%). Sixteen participants (12.2%) had patellar tendon involvement, and 14 (10.7%) had triceps tendon involvement. Of the 28 participants with hyperechoic aggregates in at least 1 tendon, 14 had asymptomatic MSU crystal deposition at 1 or both first MTP joints. The presence of MSU crystal deposition at either first MTP joint was associated with the presence of hyperechoic aggregates in at least 1 tendon (OR 3.12 [95% CI 1.31–7.42]). This association was statistically significant after adjustment for covariates (ORadj 2.96 [95% CI 1.17–7.49]). Subjects with asymptomatic MSU crystal deposition had higher serum urate levels than those without (mean difference 0.54 mg/dl [95% CI 0.12–0.96]). The prevalence of asymptomatic MSU crystal deposition at either first MTP joint increased from 0% to 24.2%, 39.1%, 29.6%, 44.4%, and 50%, respectively, in participants with serum urate levels of <5, 5–5.99, 6–6.99, 7–7.99, 8–8.99, and ≥9 mg/dl (Table 1). Other disease, demographic, and laboratory parameters were comparable between the 2 groups (Table 1). MSU crystal deposition was associated with increasing serum urate level (OR 1.50 [95% CI 1.06–2.11], ORadj 1.61 [95% CI 1.10–2.36] for each 1‐mg/dl increase in serum urate). However, there was no association between MSU crystal deposition and uric acid underexcretion status (FEUA ≤6.6%) (OR 0.71 [95% CI 0.29–1.71], ORadj 0.78 [95% CI 0.31–1.98]). Among the 117 participants with serum urate levels of ≥5 mg/dl (the cutoff value above which MSU crystal deposits were found in this study), the prevalence of asymptomatic MSU crystal deposition was 33.33%, and this increased numerically from ages in the 20s to the 40s before stabilizing (see Supplementary Table 1, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40572/abstract). However, the increase was not statistically significant, and participants age >40 years were not significantly more likely to have asymptomatic MSU crystal deposition than those ≤40 years of age (OR 1.32 [95% CI 0.59–2.95], ORadj 1.69 [95% CI 0.34–8.47]). There was no association between hyperuricemia and tendon hyperechoic deposits, and, in those with serum urate levels ≥5 mg/dl, there was no association between increasing age and tendon hyperechoic deposits (Supplementary Tables 2 and 3, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40572/abstract). Self‐reported data on age at onset of gout were available for 60 parents. The median age at onset was 53 years. On univariate analysis, there was no association between parental gout onset at or before 53 years of age and asymptomatic MSU crystal deposition (with parental gout onset after the age of 53 years as the referent) (OR 2.64 [95% CI 0.79–8.87]). However, this approached significance after adjustment for covariates (ORadj 4.14 [95% CI 0.88–19.46], P = 0.07). Discussion This study demonstrates that the sons of patients with gout have a higher prevalence of hyperuricemia 11, uric acid underexcretion 8, and asymptomatic MSU crystal deposition than observed in previous studies in which participants were preselected according to their serum urate level 4-6. The results also raise the possibility that MSU crystal deposition occurs initially in the first MTP joints and in tendons before appearing in other joints such as the ankle and the knee. However, this observation is limited by the cross‐sectional study design. It was surprising that 1 in 5 subjects with serum urate levels between 5 and 6 mg/dl had ultrasonographic features of MSU crystal deposition at the first MTP joints. This observation must be interpreted with caution as it is based on a single serum urate measurement, and it is possible that serum urate levels in these participants were higher at a previous time. However, it raises the possibility that the threshold for MSU crystal deposition in vivo may be lower than that estimated from laboratory studies. This may be due to the fact that the saturation point of urate reduces from 6.75 mg/dl at 37°C to between 4.5 and 6 mg/dl at 30–35°C, the mean temperature of the human big toe in temperate climates 12, 13. While MSU crystals were present in subjects with serum urate levels of 5–6 mg/dl, we did not find ultrasonographic evidence of MSU crystal deposition in those with levels below 5 mg/dl. This raises the possibility that the target serum urate level for treat‐to‐target urate‐lowering therapy should be <5 mg/dl, at least in individuals who continue to have gout flares despite serum urate levels between 5 and 6 mg/dl. However, further prospective studies are needed before such a strategy can be recommended. A lower‐than‐expected serum urate level in sons of patients with gout might also be explained in part by inherited tissue factors (either an increase in promoters or decrease in inhibitors) that enhance MSU crystal deposition at relatively low serum urate levels. The present findings suggest that MSU crystal deposition begins early (in the third decade of life) and becomes more prevalent with increasing age. The reduction in prevalence of MSU crystal deposition in subjects older than 60 years could be due to the sampling for this study, as people older than 60 years with MSU crystal deposits are likely to have developed gout flares, which would have excluded them from the study population. We observed intratendinous hyperechoic deposits in 35.9% of participants with MSU crystal deposits elsewhere. This is consistent with previous reports of tendon involvement in gout 14, 15. As shown in Table 2, a substantial proportion of tendon hyperechoic deposits occurred in subjects without ultrasonographic features of MSU crystal deposition in the first MTP joints. Further research, e.g., using dual‐energy computed tomography, is therefore needed to confirm the composition of these tendinous deposits before their presence can be used to imply MSU crystal deposition in the absence of a double contour sign or intraarticular tophi in other joints. Our results indicate that ultrasonographic evaluation of both first MTP joints is sufficient to identify all individuals with MSU crystal deposition. Thus, men at a high risk of gout (e.g., those with a positive family history) could undergo serum urate measurement and ultrasonography of both first MTP joints to screen for MSU crystal deposition. While ultrasonographic examination of multiple peripheral joints is time consuming, assessment of both first MTP joints takes 10–15 minutes and may make it possible for asymptomatic MSU crystal deposits to be diagnosed, in turn allowing consideration of lifestyle changes to prevent development of symptomatic gout and associated consequences. Initiation of prophylactic pharmacologic urate‐lowering treatment at this early stage would be considered controversial given the absence of symptoms and the possibility that in many people with asymptomatic MSU crystal deposition, gout flares would not develop. Such a screening strategy would require ultrasonography of 3 individuals with a serum urate level of ≥5 mg/dl to detect 1 person with asymptomatic MSU crystal deposition. However, in the absence of prospective studies evaluating the relationship between asymptomatic MSU crystal deposition and symptomatic gout, the benefit from such a strategy remains unproven. Our data also suggest that parents with younger‐onset gout are more likely to pass on the trait to their sons, although this association was not statistically significant and requires further investigation in a study with a larger sample size. There are several caveats to this study. First, the response rate was low, and it is possible that patients with severe, troublesome gout were more likely to pass on the study packets to their sons, or that sons with lifestyle risk factors were more likely to agree to participate. This raises the possibility of selection and response bias. However, the mean ± SD age at gout onset in parents of sons who participated and for whom data on the age at gout onset were available (n = 60) was 52 ± 13.65 years, and they reported a mean ± SD of 1.33 ± 2.10 gout flares in the 12‐month period preceding their original research visit. These parents also had a low comorbidity burden, with a median of 1 cardiovascular or renal comorbidity (interquartile range 1–2). Second, we did not perform joint aspiration to confirm the validity of our findings. However, in subjects with hyperuricemia, ultrasonographic changes have 100% sensitivity and 88% specificity for MSU crystal deposition, compared to joint aspiration 5. Finally, we measured serum urate only on a single occasion. In conclusion, the results of this study demonstrate that asymptomatic sons of patients with gout frequently have hyperuricemia and uric acid underexcretion, and have a high prevalence of MSU crystal deposition. This suggests that screening of such individuals and discussion of early management, involving addressing modifiable risk factors (overweight, obesity, high fructose intake, etc.) in order to reduce their risk of developing symptomatic gout, should be considered. Acknowledgments The authors would like to acknowledge the study participants and their parents. Author Contributions All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Abhishek had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design Abhishek, Courtney, Jones, Zhang, Doherty. Acquisition of data Abhishek, Courtney, Jenkins. Analysis and interpretation of data Abhishek, Sandoval‐Plata. Supporting Information References Notes : Drs. Abhishek and Doherty's work was supported by an investigator‐initiated departmental research grant from AstraZeneca and Ironwood Pharmaceuticals. Ms Sandoval‐Plata's work was supported by a PhD scholarship from Consejo Nacional de Ciencia y Tecnología, Mexico. Dr. Abhishek has received speaking fees from Menarini (less than $10,000) and research grants from AstraZeneca and Oxford Immunotec. Dr. Zhang has received speaking fees and/or honoraria from AstraZeneca, Grünenthal, Bioiberica, and Hisun (less than $10,000 each). Dr. Doherty has received consulting fees and/or honoraria from AstraZeneca, Grünenthal, Mallinkrodt, and Roche (less than $10,000 each) and research grants from AstraZeneca and Oxford Immunotec. 3 All participants with asymptomatic monosodium urate monohydrate (MSU) crystal deposition had first metatarsophalangeal joint involvement. IQR = interquartile range; eGFR = estimated glomerular filtration rate; FEUA = fractional excretion of uric acid. 4 Eleven participants were prescribed antihypertensive drugs; 1 received bendroflumethiazide. 5 Six participants were prescribed statins. 6 Both participants were prescribed oral hypoglycemic drugs. 7 P = 0.01 versus participants without asymptomatic MSU crystal deposition. 8 One participant had a double contour sign at 1 ankle; triangular fibrocartilage involvement was not observed in any participant. Values are the number (%) of joints/tendons. MSU = monosodium urate monohydrate; MTP = metatarsophalangeal. Citing Literature Number of times cited: 1 Xiao Chen, Zhongqiu Wang, Na Duan, Wenjing Cui, Xiaoqiang Ding and Taiyi Jin, The benchmark dose estimation of reference levels of serum urate for gout, Clinical Rheumatology, 10.1007/s10067-018-4273-1, (2018). Crossref

|
Scooped by
Gilbert C FAURE
onto Rheumatology-Rhumatologie November 17, 2018 5:09 AM
|
No comment yet.
Sign up to comment



 Your new post is loading...
Your new post is loading...