Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
December 8, 2024 4:06 AM
|
This eLearning course has been designed to educate healthcare professionals (HCPs) on the management of rheumatoid arthritis with interleukin-6 receptor inhibition therapy, with a specific focus on tocilizumab and its biosimilars.

|
Scooped by
Gilbert C FAURE
December 20, 2023 8:37 AM
|
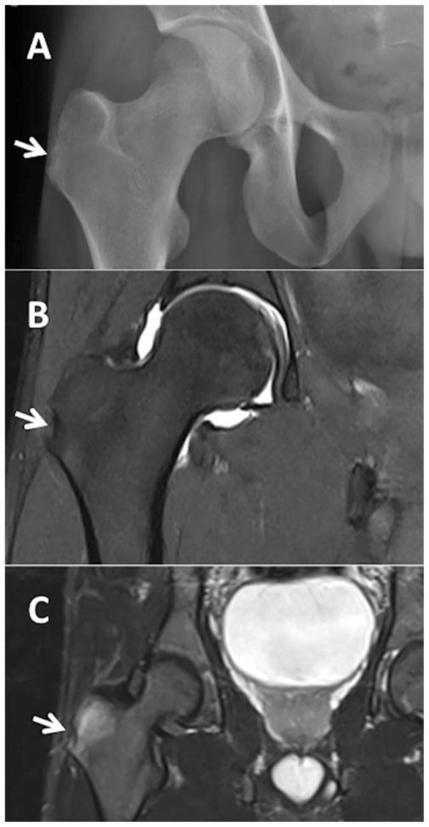
WHAT IS ALREADY KNOWN ON THIS TOPICMonoclonal therapy for one inflammatory disease may paradoxically trigger another inflammatory disease.Recent case reports have implicated an association between anti-IL-5 (IL, interleukin) antibody therapy used to treat severe asthma and the development of rheumatoid arthritis (RA).WHAT THIS STUDY ADDSOut of 142 patients within our asthma service taking anti-IL-5 antibody therapy for at least 1 month, and with a mean duration of 3.5 years on therapy, only one developed RA suggesting that RA is a relatively uncommon complication in the short-medium term.HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICYTreating clinicians should be mindful of the possibility of developing inflammatory arthritis following the initiation of anti-IL-5 therapy and ensure appropriate review and assessment should their patient develop arthralgia as, while uncommon, these could represent a significant source of morbidity.IntroductionThere has been a wide adoption of monoclonal antibody therapy in rheumatology, respiratory medicine, and an increasing number of specialties for the treatment of many inflammatory diseases. Of particular interest is that monoclonal therapy for one inflammatory disease may paradoxically trigger another inflammatory disease. Pertinent examples include tumour necrosis factor inhibitor therapy triggering multiple sclerosis,1 interleukin 17 (IL-17) therapy for psoriasis linked to inflammatory bowel disease2 and more recently IL-4/13 blockade used for atopic dermatitis being associated with de novo psoriasis and arthritis.3 4Arthralgias are a known adverse effect of anti-IL-5 biologics,5 6 however, a few recent case reports have found this association may extend to inflammatory arthritis such as RA.7 8 The prevalence of these findings across a wider cohort of patients remains relatively unknown. Here we present an audit from a large, single-centre’s severe asthma service which looks for the prevalence of RA across all patients being treated with mepolizumab and benralizumab, two commonly used anti-IL-5 therapies.MethodsAll patients with severe eosinophilic asthma across the Leeds Teaching Hospitals NHS Trust’s (LTHT) Respiratory Service, who had received at least 1 month of mepolizumab or benralizumab therapy, were included in this clinical audit.Each patient’s electronic records, including hospital records, clinic letters, general practitioner (GP) records and electronic pathology results were searched. We recorded whether patients had presented with any signs or symptoms of synovitis (eg, joint pain, swelling and tenderness) either prior- or post-commencing biologics, whether their serology (rheumatoid factor (RF) and/or anti-CCP antibody (ACPA)) and acute phase reactants (C- Reactive Protein (CRP) and/or Erythrocyte Sedimentation Rate (ESR)) had been measured and the timing and duration of their symptoms. Using this information, we then calculated the number of points each patient with symptoms would score on the ACR/EULAR 2010 Rheumatoid Arthritis classification criteria.9 We also recorded the dose of routine steroids the patients were receiving prior to starting biologics, and whether they were weaned off steroids within 1 year of commencing biologics. Finally, we recorded whether the patients had been seen in our early arthritis clinic and received a formal diagnosis on an inflammatory arthritis.ResultsA total of 142 patients (57 males, 85 females and mean age 58.2 years old) were being treated with anti-IL-5 biologics under the LTHT’s severe asthma clinic, with a mean duration of 3.5 years on therapy. Eighty-nine were on mepolizumab and 53 on benralizumab. The mean daily dose of steroids prior to starting anti-IL-5 therapy was 6.0 mg prednisolone, reducing to 3.1 mg at 1 year post-therapy. Seventy-five patients were steroid-free after 1 year of therapy.Only one patient among 500 patient years of exposure to anti-IL-5 therapy received a formal diagnosis of RA suggesting an overall annual incidence of 20 cases per 10 000 patients (95% CI 2.8 to 142). This man in his 70s presented to our early arthritis clinic 18 months after having been started on mepolizumab for his severe eosinophilic asthma. Prior to starting biologics, his asthma had been poorly controlled with salbutamol, budesonide/formoterol combination inhaler, tiotropium inhalers and daily low-dose oral corticosteroids. Within weeks, he developed symmetrical arthralgia involving small joints, particularly his wrists and knuckles, as well as significant early morning stiffness lasting more than 1 hour. On examination, he had clinical synovitis in the wrists and metacarpophalangeal joints bilaterally, as well as right shoulder capsulitis with limiting range of motion.Blood tests revealed a raised CRP of 24 mg/L, White Cell Count (WCC) 8.77 10 × 9 /L, RF of 263.2 iu/mL (normal<14.0) and an ACPA of >300 U/mL (normal<2.99). Ultrasound imaging of the hands and wrists showed bilateral grade II grey scale with grade II power Doppler (figure 1) with bilateral wrist erosions. There was hypoechogenicity of the left extensor carpi ulnaris tendon with some associated grey scale and power Doppler. MRI of the left hand revealed extensive subchondral bone marrow oedema (figure 1) and multiple erosions across all carpal bones and carpometacarpal joints (figure 1).<img width="342" alt="Figure 1" height="440" class="highwire-fragment fragment-image" src="https://rmdopen.bmj.com/content/rmdopen/9/4/e003583/F1.medium.gif">Download figure Open in new tab Download powerpoint Figure 1 (A) Fat suppression MRI of the left wrist and MCPs showing extensive bone oedema (white arrows) and joint effusion (black asterisk). (B) T1-weighted MRI of the left wrist showing diffuse erosions of the left wrist (white arrows). (C) Longitudinal ultrasound image showing synovitis of the right wrist with grey scale (white asterisk) and power Doppler (white arrow).He was diagnosed with RA as per the American College of Rheumatology (ACR)/EULAR classification criteria and started on prednisolone 10 mg daily to control the inflammation, followed by sulfasalazine 1 month later as the disease modifying agent. He was followed-up in rheumatology clinic 2 months later and showed significant improvements: the joint pain and swelling had settled, and while he still experienced early morning stiffness, this was less debilitating. His inflammatory markers had also resolved with CRP<5.0 mg/L and WCC 9.31 10 × 9 /L.Of the remaining 141 patients, 16 developed bilateral polyarthralgia of greater than 1 month duration (eight mepolizumab and eight benralizumab), with a median onset of 12 months after commencing a biological therapy. Of these patients 9/16 were tested for RF and ACPA and in all cases, their serology was negative; 15/16 patients had acute phase inflammatory markers measured and these were only elevated in three patients. All 16 of these patients were on a maintenance dose of prednisolone prior to starting the biologic (mean dose 10.1 mg/day), with 10 of them completely weaned off steroids within 12 months.Using the information available from the patient’s electronic records, the mean number of points scored on the ACR/EULAR RA criteria was 3.2 (range 1–6). The patient who scored six points was reviewed in the early arthritis clinic and the symptoms were felt to be more in keeping with osteoarthritis than an inflammatory arthritis. Similarly, none of the other patients had received a confirmed diagnosis of inflammatory arthritis by either their GP or by a rheumatologist.Only one other patient became newly RF positive (17.1 iu/mL), 1 month after commencing mepolizumab; however, this seemed to be an incidental finding as the patient had a broad set of bloods taken while admitted to the intensive care unit for a severe exacerbation of asthma, and at no point since has complained of rheumatological symptoms.We were unable to access the GP records for 37 patients and as such could not review whether they had presented to their GPs with new rheumatological symptoms. However, we were able to access their pathology test records electronically and found no evidence of positive RA serology in any of these patients and no rheumatological referrals to our centre that has a well-developed early RA network.DiscussionThere is an emerging interest in IL-5 blockade and the potential development of RA. We present a single-centre’s experience of 500 patient years on anti-IL-5 monoclonal antibody exposure therapy for severe asthma.As expected, arthralgias were a relatively common side-effect of anti-IL-5 therapy. As for progression to RA, we found only one convincing case. While relatively low, the implied annual incidence of 20 cases per 10 000 patients is several fold higher than the annual incidence of RA in the UK (1.5 per 10 000 men and 3.6 per 10 000 women).10 Given the wide CIs, however, no firm conclusions can be offered in relationship to our single case and to the relative risk of RA following anti-IL-5 therapy.A major confounding variable is the weaning of steroids in most patients started on biologics. This poses a challenge in associating the development of symptoms with the initiation of the anti-IL-5 therapy, as opposed to the withdrawal of steroids unmasking a pre-existing disease. Additionally, one must consider whether the risk of developing RA is modified by the underlying condition, and indeed there is some evidence to suggested that asthma may be positively associated with RA.11 However, these population-based studies look at asthma as a whole, rather than divided into its endotypes (eg, eosinophilic vs neutrophilic asthma) and as such these have not yet challenged the conventional belief that Th1 and Th2 diseases are inversely related.Emerging evidence has implicated a core role for regulatory eosinophils (rEos) in the resolution of RA.12 In murine models of RA, the expansion of rEos in the synovial fluid as a by-product of inducing eosinophilic asthma was sufficient in bringing about remission of arthritis, and inhibiting the IL-5 pathway would subsequently induce relapse of the arthritis.12 Further evidence supporting a role for rEos in RA can be found at a genetic level where Eotaxin-3, one of the main drivers of eosinophil recruitment, has single nucleotide polymorphisms associated with RA13 and from studying the role of IL-5 in Th2 responses to Helminth infections,14 with mouse models of RA also identifying Helminth infections as protective.15 Hence, the suggestion that the expansion of eosinophils in the synovium ‘regulate’ the proinflammatory Th1 pathways driving synovial inflammation.12 This invites the notion that in a patient with subclinical, yet endogenously controlled, synovial inflammation, removing rEos by administering anti-IL-5 therapeutics may tip the balance in favour of inflammation and permit symptomatic disease. However, if there is little proinflammatory Th1 synovial activity in the first place, then inhibiting rEos with anti-IL-5 biologics may be insufficient to precipitate an inflammatory arthritis.Interestingly, there is debate as to whether rEos are depleted to varying degrees depending on the anti-IL-5 biologic used. In mice, inflammatory eosinophils (iEos)—the primary targets of anti-IL-5 biologics in asthma—may be dependent on IL-5 for activity, whereas rEos may not be.16 This would suggest that benralizumab, a high-affinity IL-5 receptor antagonist,17 would deplete both iEos and rEos through NK-mediated killing, whereas mepolizumab, an anti-IL-5 monoclonal antibody,17 may deplete iEos but keep rEos intact. However, this idea has recently been challenged with evidence that anti-IL-5 treatment depletes all populations of eosinophils.18 Whether this distinction would result in a different pattern of adverse effects in patients remains unclear, notably as the patient who developed RA in this report was receiving mepolizumab.As an audit, this study serves to identify the prevalence of a relatively rare complication of anti-IL-5 therapy. We were unable to find clear evidence for a pattern of emergent RA nor other inflammatory arthritis in our cohort of 142 patients. Further studies may be required to characterise the nature and significance of these findings in clinical groups and to identify whether there is an actual association between novel anti-IL-5 biologics and RA.Data availability statementThe data that support the findings of this study are available upon reasonable request.Ethics statementsPatient consent for publicationConsent obtained directly from patient(s).Ethics approvalThis study was registered as a clinical audit and given the retrospective nature of the data collection process did not require formal ethical approval. In completing this audit, full ethical standards were upheld in accordance with the principles of clinical governance. From the one patient whose details were discussed in more detail we have gained full written consent.References↵Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 2001;57:1885–8. doi:10.1212/wnl.57.10.1885OpenUrlCrossRefPubMed↵Hohenberger M, Cardwell LA, Oussedik E, et al. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat 2018;29:13–8. doi:10.1080/09546634.2017.1329511OpenUrlPubMed↵Bridgewood C, Newton D, Bragazzi N, et al. Unexpected connections of the IL-23/IL-17 and IL-4/IL-13 cytokine axes in inflammatory arthritis and enthesitis. Semin Immunol 2021;58:101520. doi:10.1016/j.smim.2021.101520OpenUrl↵Bridgewood C, Wittmann M, Macleod T, et al. T helper 2 IL-4/IL-13 dual blockade with dupilumab is linked to some emergent T helper 17‒Type diseases, including seronegative arthritis and enthesitis/enthesopathy, but not to humoral autoimmune diseases. J Invest Dermatol 2022;142:2660–7. doi:10.1016/j.jid.2022.03.013OpenUrl↵Harrison T, Canonica GW, Chupp G, et al. Real-world Mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J 2020;56:2000151. doi:10.1183/13993003.00151-2020↵Liu W, Ma X, Zhou W. Adverse events of benralizumab in moderate to severe eosinophilic asthma: a meta-analysis. Medicine (Baltimore) 2019;98:e15868. doi:10.1097/MD.0000000000015868↵Kawabata H, Satoh M, Yatera K. Development of rheumatoid arthritis during anti-Interleukin-5 therapy in a patient with refractory chronic eosinophilic pneumonia. J Asthma Allergy 2021;14:1425–30. doi:10.2147/JAA.S342993OpenUrl↵Dupin C, Morer L, Phillips Houlbracq M, et al. Arthritis, a new adverse effect of anti-Il5 Biologics in severe asthma patients. European Respiratory Journal 2022;60:2432. doi:10.1183/13993003.congress-2022.2432OpenUrlCrossRef↵Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American college of rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. doi:10.1002/art.27584OpenUrlCrossRefPubMedWeb of Science↵NICE guideline. Overview: rheumatoid arthritis in adults: management [Guidance, NICE]. 2018. Available: https://www.nice.org.uk/guidance/ng100 [Accessed 25 Sep 2023].↵Rolfes MC, Juhn YJ, Wi C-I, et al. Asthma and the risk of rheumatoid arthritis: an insight into the heterogeneity and phenotypes of asthma. Tuberc Respir Dis (Seoul) 2017;80:113–35. doi:10.4046/trd.2017.80.2.113OpenUrl↵Andreev D, Liu M, Kachler K, et al. Regulatory eosinophils induce the resolution of experimental arthritis and appear in remission state of human rheumatoid arthritis. Ann Rheum Dis 2021;80:451–68. doi:10.1136/annrheumdis-2020-218902OpenUrlAbstract/FREE Full Text↵Guellec D, Milin M, Cornec D, et al. Eosinophilia predicts poor clinical outcomes in recent-onset arthritis: results from the ESPOIR cohort. RMD Open 2015;1:e000070. doi:10.1136/rmdopen-2015-000070↵Mishra PK, Palma M, Bleich D, et al. Systemic impact of intestinal helminth infections. Mucosal Immunol 2014;7:753–62. doi:10.1038/mi.2014.23OpenUrlCrossRefPubMed↵Osada Y, Shimizu S, Kumagai T, et al. Schistosoma Mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol 2009;39:457–64. doi:10.1016/j.ijpara.2008.08.007OpenUrlCrossRefPubMed↵Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 2016;126:3279–95. doi:10.1172/JCI85664OpenUrlCrossRefPubMed↵Caminati M, Menzella F, Guidolin L, et al. Targeting eosinophils: severe asthma and beyond. Drugs Context 2019;8:212587. doi:10.7573/dic.212587OpenUrl↵Dolitzky A, Grisaru-Tal S, Avlas S, et al. Mouse resident lung eosinophils are dependent on IL-5. Allergy 2022;77:2822–5. doi:10.1111/all.15362OpenUrl

|
Scooped by
Gilbert C FAURE
April 5, 2023 4:19 AM
|
Glucocorticoids are recommended for short-term use in rheumatoid arthritis (RA), but many patients remain on long-term therapy. We evaluated the variability in glucocorticoid prescribing across rheumatologists to inform interventions to limit long-ter

|
Scooped by
Gilbert C FAURE
September 24, 2021 2:24 PM
|
In the last of our series showcasing our Best Practice Award winners, we talk to the rheumatology team at North Bristol NHS Trust, who developed an education programme to help support arthritis patients. The rheumatology team at North Bristol NHS Trust won their Best Practice Award for their Living Well Pathway, developed to help patients living with inflammatory arthritis. The course was designed to support both physical and psychological wellbeing, empowering patients to feel more in control of their health. The project The project was set up to incorporate NICE guidelines, which state that patients should be offered education and self-management within one month of diagnosis and should be helped to adjust to their condition. The pathway is split into two parts; a Living Well event for newly diagnosed patients and a Living Well course for people who have been diagnosed for a year or more. Clinical psychologist Kate Druett, who helped set the programme up, said: “We wanted to make sure all our patients were aware of the different services available within our team to support them. We also wanted to be holistic and supportive in terms of both physical and emotional wellbeing.” The Living Well event has seen 77 patients attend since it began in March 2017: It's signposting and information session giving patients the opportunity to meet the rheumatology team and other patients with inflammatory arthritis During a series of interactive talks, patients can ask questions and find out what support is available, as well as what might help with managing their condition These run for 2.5 hours every other month and alternate between a morning or evening slot so it’s accessible for people who work. Attendees have found the inclusion of a talk by a patient volunteer particularly valuable; this provides a picture of their own experience, a talk about what they have found helpful in learning to live with the condition, and offers hope for the future. The Living Well course was completed by 56 patients between October 2017-September 2019. It: Is aimed at helping patients who are at least one-year post diagnosis cope with their symptoms Weekly course, seven sessions of 2.5 hours each Includes a two-month 'reunion' follow-up Covers issues like fatigue, stress, keeping active, and goal-setting. Kate explains: “We interchange who runs the course amongst the team, but we have representatives from Specialist Nursing, Occupational Therapy and Psychology.” Another key element is the social aspect and patients supporting each other in the community once the course comes to an end. Kate says: “Patients are encouraged to meet informally amongst themselves to help keep the momentum going and put what they've learnt into practice.” Following the course, many patients have reported an increase in confidence in knowing where to get help with their condition and have seen improvements in their mood. Kate says: “One of the key benefits we’ve seen is that people report a lift in their confidence. Before the course patients may have been struggling with fatigue or pain for some time and lost a lot of their confidence. They often tell us they feel low and isolated, finding day-to-day activities difficult, and often feel quite stuck.” She continues: “It’s been rewarding to hear how people have really valued the opportunity to meet and learn from others. Patients have often made many changes, such as taking regular time for themselves to swim or get back to day-to-day activities they used to enjoy, such as cooking. Others made changes at work so that it's more manageable, or made plans to return to work.” The team use an active and supportive group of volunteers to help them run the signposting event. Feedback and discussion with previous participants help with continued reviewing of the course content Kate explains: “They've contributed to the content based on their experience of what would have been helpful to them when they were diagnosed. We know people find strength and encouragement from hearing from other patients who have been through similar circumstances.” What happens next? Post-pandemic, the team will restart the courses and will look at putting some of the content online. Kate says: “We’ve recorded some short films with our participants so that they can be used on our website to encourage other patients to get involved. Plus, we want to get some of the other useful information online.”. What did the judges say? The judges commended the team for helping patients take control of their health and have better outcomes. BSR’s Chief Executive, Ali Rivett, says: “I was particularly impressed by how patient-focused this initiative was. Patients are involved in the design and delivery of the programme, alongside their AHP team. Participant feedback is positive, with reported increases in patient activation measures alongside other key metrics.” Congratulations to all our winners, who are working hard to improve the lives of patients. Best Practice Awards

|
Scooped by
Gilbert C FAURE
June 1, 2021 3:48 AM
|
EULAR has published evidence-based recommendations on the use of intra-articular therapies (IAT) based on the literature review and recommendations of a multidisciplinary international task force. These IAT recommendations apply to adult patients with peripheral arthropathies.
The committee published 5 overarching principles and 11 recommendations addressing procedure and setting, accuracy, routine and special aseptic care, safety issues, precautions, special populations, repeated joint injections, local anaesthetics use and IAT aftercare.

|
Scooped by
Gilbert C FAURE
February 2, 2021 3:58 AM
|
Research ArticleAutoimmunity Free access | 10.1172/JCI93450 Two rheumatoid arthritis–specific autoantigens correlate microbial immunity with autoimmune responses in joints Annalisa Pianta,1 Sheila L. Arvikar,1 Klemen Strle,1 Elise E. Drouin,1 Qi Wang,2 Catherine E. Costello,2 and Allen C. Steere1 Published June 26, 2017 - More info View PDF Abstract In rheumatoid arthritis (RA), immunological triggers at mucosal sites, such as the gut microbiota, may promote autoimmunity that affects joints. Here, we used discovery-based proteomics to detect HLA-DR–presented peptides in synovia or peripheral blood mononuclear cells and identified 2 autoantigens, N-acetylglucosamine-6-sulfatase (GNS) and filamin A (FLNA), as targets of T and B cell responses in 52% and 56% of RA patients, respectively. Both GNS and FLNA were highly expressed in synovia. GNS appeared to be citrullinated, and GNS antibody values correlated with anti–citrullinated protein antibody (ACPA) levels. FLNA did not show the same results. The HLA-DR–presented GNS peptide has marked sequence homology with epitopes from sulfatase proteins of the Prevotella sp. and Parabacteroides sp., whereas the HLA-DR–presented FLNA peptide has homology with epitopes from proteins of the Prevotella sp. and Butyricimonas sp., another gut commensal. Patients with T cell reactivity with each self-peptide also had responses to the corresponding microbial peptides, and the levels were directly correlated. Furthermore, HLA-DR molecules encoded by shared-epitope (SE) alleles were predicted to bind these self- and microbial peptides strongly, and these responses were more common in RA patients with SE alleles. Thus, sequence homology between T cell epitopes of 2 self-proteins and a related order of gut microbes may provide a link between mucosal and joint immunity in patients with RA. Introduction Rheumatoid arthritis (RA) is an HLA class II–associated autoimmune disease, in which arthritogenic T cells drive the progressive inflammation and destruction of synovial joints (1). Both genetic and environmental factors are thought to contribute to disease development and progression. The greatest genetic risk factor is HLA-DRB1–susceptibility alleles that share a 5–amino acid sequence in the B1 chain, termed the RA shared epitope (SE) (2). HLA-DRB1 SE alleles largely influence the development of seropositive RA, which is defined by positive tests for rheumatoid factor (RF) and/or anti–citrullinated protein antibodies (ACPAs) (3), the latter being the only known specific autoantibodies for this disease (4–6). RF and/or ACPAs may develop years before the onset of clinical arthritis (7–9), suggesting that autoimmunity may be triggered at sites other than joints in patients with RA. Causative environmental factors are less well characterized. However, T cell epitope mimicry between microbial pathogens and self-proteins has been implicated as a possible factor in the induction or exacerbation of autoimmune disease (10–12). In addition, alteration of the oral or gut microbiota may affect mucosal immunity, inducing aberrant immune responses that affect joints in patients with RA (13, 14). Using high-throughput sequencing, Scher et al. showed that Prevotella species (spp.), including P. copri, in the gut microbiota were expanded in stool samples from patients with new-onset RA (NORA), suggesting that these organisms might have this role in RA pathogenesis (13). Moreover, a recent study in mice showed that gut dysbiosis contributes to arthritis development via the activation of autoreactive T cells in the intestine (15). Proposed mechanisms to link infection and autoimmunity include molecular mimicry between T cell microbial and host epitopes (16); infection-induced alteration and release of sequestered self-antigens (11); or nonspecific, infection-induced inflammatory responses that function as adjuvants in the induction of pathogenic autoimmunity (17, 18). The identification of disease-relevant infectious or self-antigens has been challenging in any autoimmune disease, but current discovery-based methods offer innovative approaches to this problem. We have developed an approach for antigen detection in chronic inflammatory arthritides, in which HLA-DR–presented peptides (T cell epitopes) are identified directly from patients’ inflamed synovial tissue, synovial fluid mononuclear cells (SFMCs), or peripheral blood mononuclear cells (PBMCs) by liquid chromatography–tandem mass spectrometry (LC-MS/MS) and tested for immunogenicity using patients’ samples (19–24). With this approach, we recently identified an HLA-DR–presented peptide from a 27-kDa protein of P. copri (Pc-p27), which stimulated T and B cell responses in approximately 40% of patients with RA, but not in patients with other rheumatic diseases or in healthy controls (HCs) (25). Using the same methodology, we report here the identification of 2 previously unidentified autoantigens, N-acetylglucosamine-6-sulfatase (GNS) and filamin A (FLNA) that are targets of T and B cell responses and appear to be specific for RA. Both autoantigens are highly expressed in inflamed synovial tissue; they share homologous T cell epitopes with Prevotella and several other gut microbes, and they are targets of specific T and B cell responses in patients with RA, providing evidence that may link immune responses to microbial peptides from gut commensals and autoimmune responses affecting joints. Results Identification of naturally presented HLA-DR peptides (T cell epitopes). In a recent study (20), we identified HLA-DR–presented peptides in synovial tissue, SFMCs, and PBMCs from 5 patients with RA using LC-MS/MS, and the immunogenicity of the peptides was determined using patients’ samples in enzyme-linked immunospot (ELISpot) assays. The findings from 1 patient (referred to here as RA1) were of particular interest. She had classic, seropositive RA, with severe symmetrical polyarthritis, a positive test for ACPAs, and 2 copies of SE alleles (HLA-DRB1*0401 and *0101). In this patient, an immunogenic HLA-DR–presented peptide derived from a P. copri protein (Pc-p27) was identified from the patient’s PBMCs (25). We then showed that approximately 40% of patients with RA have T and/or B cell responses to Pc-p27 or to the whole P. copri organism (25). In patient RA1, 2 immunogenic HLA-DR–presented human self-peptides derived from GNS and FLNA were also identified from her synovial tissue, and the same FLNA peptide was also found in her PBMCs (20). The HLA-DR–presented peptide derived from GNS was predicted to be promiscuous, binding to 24 of the 25 HLA-DR molecules modeled in the program TEPITOPE (26), and the FLNA-derived peptide was predicted to bind 9 of the 25 HLA-DR molecules. With both peptides, this included binding by HLA-DR molecules encoded by SE alleles *0101, *0401, *0404, and *0405. Neither the GNS protein, nor the FLNA protein, nor the P. copri protein had previously been noted to be antigens in RA. T cell reactivity to GNS and FLNA peptides. To determine the immunogenicity of HLA-DR–presented peptides and their source proteins more broadly, we have developed a cohort of NORA patients seen prior to commencing therapy with disease-modifying antirheumatic drugs (DMARDs), which is a time when immune responses would be expected to be most robust. For comparison, we tested samples from patients with Lyme arthritis (LA) and from HC subjects. HLA-DR typing showed that 60% of the 40 RA patients had SE alleles, and 50% of the 10 LA patients and 42% of the 15 healthy subjects also had SE alleles. Nevertheless, since the patients and HCs had a range of different HLA-DR alleles, and since cell numbers are limited in human patients, our initial approach for determining T cell responses in multiple individuals consisted of pooling the original peptide with 3 additional peptides from the same protein that are predicted by the program TEPITOPE to be promiscuous HLA-DR binders (26). In addition, because of a limited number of cells, we did not include the testing of irrelevant control peptides in these experiments. However, we have previously shown that patients with RA do not have reactivity to peptides derived from endothelial cell growth factor (ECGF) or MMP-10 peptides (21, 23). These autoantigens in LA are irrelevant in RA. When PBMCs from 40 patients with NORA were stimulated with the GNS peptides, we found that 14 of the 40 patients (35%) secreted levels of IFN-γ that were greater than 3 SD above the mean value for HCs (P = 0.006), as determined by an IFN-γ/IL-17 double-color ELISpot assay (Figure 1A). In comparison, PBMCs from patients with LA lacked reactivity to these peptides (P = 0.005). When FLNA peptides were used to stimulate PBMCs from the same set of patients and control subjects, 17 of the 40 patients with NORA (42%) had IFN-γ levels that were greater than 3 SD above the mean value for HCs (P = 0.001) and for patients with LA (P = 0.0005) (Figure 1B). In patients with RA, the predominant response to stimulation with both peptide sets was a Th1-type response with IFN-γ secretion, whereas PBMCs from only 3 RA patients secreted IL-17 (data not shown). Altogether, 21 of the 40 patients (52%) had T cell reactivity to GNS and/or FLNA peptides, and 10 (25%) had reactivity to both. Figure 1 T cell reactivity to GNS and FLNA peptides in RA patients and comparison group subjects. In initial experiments, (A) PBMCs from patients with RA or LA or from HC subjects were stimulated with a pool of 4 peptides, including the single GNS HLA-DR–presented peptide isolated from the synovial tissue of patient RA1, and 3 predicted promiscuous HLA-DR–binding peptides from GNS (1 μM each). (B) PBMCs from patients and HCs were incubated with a pool of 4 peptides, including the single FLNA HLA-DR–presented peptide identified from the synovial tissue and PBMCs from patient RA1, and 3 predicted promiscuous HLA-DR–binding peptides from FLNA (1 μM each). In each assay, a positive control (phytohemagglutinin) and a negative control (no peptide) were included. The amount of IFN-γ secretion, as determined by an ELISpot assay, is shown. A positive response was defined as greater than 3 SD above the mean value for HCs (area above the shaded region). The values for patient RA1 are indicated with a star. Horizontal lines represent the mean values for each group. P values were determined by unpaired, 2-tailed t test with Welch’s correction. SFU, spot-forming units per million PBMCs. B cell reactivity to GNS and FLNA proteins. Since the role of CD4+ T cells would likely be to help B cells produce autoantibodies against GNS or FLNA, we examined IgG levels for these proteins in serum samples from patients with RA and control group subjects. Since sera (but not PBMCs) were also available from patients with chronic RA (CRA), testing was done in 48 NORA patients and 53 CRA patients. Because the results were similar in both groups, they are presented together here. Of the 101 patients with RA, 32 (32%) had IgG antibody responses against GNS that were greater than 3 SD above those in HCs (P < 0.0001) (Figure 2A). In contrast, none of the 106 patients with other diseases, including those with LA, spondyloarthropathy (SpA), or connective tissue diseases (CTD), and none of the 50 HC subjects had positive IgG antibody responses against the protein (in each instance, P < 0.0001). Similarly, 27 of the 101 (27%) patients with RA had levels of IgG antibodies against FLNA that were greater than 3 SD above those in HCs (P < 0.0001), whereas only 2 patients with CTD had borderline positive IgG antibody responses against FLNA, and none of the other control subjects had positive responses (Figure 2B). Altogether, 48 (48%) of the 101 RA patients had IgG autoantibodies against GNS and/or FLNA, and 10 (10%) had IgG reactivity against both proteins. Figure 2 IgG and IgA responses to GNS and FLNA in RA patients and comparison group subjects. Serum samples from 259 patients with RA, patients with other forms of chronic inflammatory arthritis, and HCs were tested by ELISA for autoantibodies. (A and C) Plates were coated with the GNS protein and incubated with serum from patients or HCs. All serum samples were tested in duplicate for anti-GNS IgG (A) or IgA (C) antibody responses. (B and D) Plates were coated with the FLNA protein and incubated with serum from patients or control subjects. All serum samples were tested in duplicate for anti-FLNA IgG (B) or IgA (D) antibody responses. For all analyses, positivity was defined as greater than 3 SD above the mean value for HCs (area above the shaded region). Symbols represent values in individual patients, and horizontal lines show the mean values. Values for patient RA1 are indicated with a star. Only significant P values, determined by unpaired, 2-tailed t test with Welch’s correction, are shown. Because autoimmune processes in RA may be triggered at mucosal sites, we also tested the levels of IgA antibodies against GNS and FLNA in serum samples from patients and control subjects. Of the 101 patients with RA, 16 (16%) had elevated IgA antibody responses against GNS that were greater than 3 SD above those in HCs (P < 0.0001) (Figure 2C). In contrast, of the 106 patients with other rheumatic diseases and the 50 HC subjects, only 1 patient with SpA had borderline positive IgA antibodies. Similarly, 15 (15%) of the 101 RA patients had FLNA IgA responses that were greater than 3 SD above those in HCs (P = 0.0002), whereas only 1 HC subject and 2 patients with LA had low-level positive responses (Figure 2D). Altogether, 21 (21%) of the 101 RA patients had IgA antibody responses against GNS and/or FLNA, and 10 (10%) had IgA antibody responses against both proteins. When IgG and IgA responses were considered together, 48 of the 101 patients with RA (48%) had IgG antibody responses against GNS and/or FLNA; 21 (21%) had IgA responses against 1 or both of the proteins; and 56 (55%) had IgG and/or IgA responses against the proteins. Of the 14 patients who had T cell responses to GNS peptides, 11 (79%) had IgG and/or IgA antibody responses against the GNS protein. Among the 17 patients who had T cell reactivity to the FLNA peptides, 6 (35%) had IgG and/or IgA antibody responses against the FLNA protein. Thus, T and B cell concordance was greater with GNS than with FLNA. Correlation of antibody responses against P. copri, GNS, and FLNA. Using these same serum samples (25), we have previously tested IgG and IgA antibody responses against 2 RA-associated bacteria, P. copri, a gut microbe, and Porphyromonas gingivalis, a periodontal pathogen (27). Antibody responses against P. copri were found in 32% of RA patients, but were absent in patients with other CTDs, SpA, or LA, as well as in healthy subjects (25). Therefore, using these data, we correlated IgG and IgA antibody responses against these 2 organisms with the GNS and FLNA antibody responses determined here. In patients with RA, the levels of anti-GNS IgG and IgA antibodies strongly correlated with P. copri antibody responses (P = 0.002 and P < 0.0001, respectively), and we found a similar correlation between anti-FLNA IgG and IgA antibody responses and P. copri antibodies (P < 0.0001 and P < 0.0001) (Figure 3A). In contrast, anti-GNS and anti-FLNA IgG or IgA levels did not correlate with P. gingivalis antibody responses (Figure 3B). Additionally, we observed no correlations among these parameters in healthy subjects. Thus, in RA patients, the higher the IgG or IgA antibody responses against P. copri, the greater the autoantibody responses against these autoantigens. Figure 3 Autoantibody correlations with P. copri and P. gingivalis antibodies. Correlations between anti-GNS or anti-FLNA antibodies (IgG or IgA) and antibodies against P. copri (A) or P. gingivalis (B) in 101 RA patients. The r and P values for the corresponding statistical comparisons were determined by Spearman’s correlation test. Testing of citrullinated GNS and FLNA proteins. Because citrullinated autoantigens are thought to play a central role in RA, particularly in patients with SE alleles, we investigated whether autoantibody responses against GNS or FLNA were greater when these proteins were citrullinated. For this purpose, the native proteins were citrullinated in vitro using recombinant human (rh) peptidylarginine deiminase 4 (PAD4) enzyme. Using samples from 46 RA patients in whom a sufficient amount of serum still remained, IgG antibody responses were higher against citrullinated GNS compared with responses against the uncitrullinated protein (P = 0.005), whereas the responses were negative against both forms of the protein in 15 HC subjects (Figure 4A). Moreover, the magnitude of anti–citrullinated GNS antibody responses correlated with ACPA levels in these patients (P = 0.03) (Figure 4B). In contrast, IgG antibody responses against citrullinated and uncitrullinated FLNA were not significantly different in the 46 patients (Figure 4C), and the levels of anti–citrullinated FLNA antibodies did not correlate with ACPA levels (Figure 4D). These results suggest that the GNS protein, but not the FLNA protein, may be citrullinated in vivo in patients with RA. Figure 4 Autoantibody responses to citrullinated GNS and FLNA, and correlations with ACPAs. Serum samples from 46 patients with RA and 15 healthy individuals were tested for IgG antibody responses against citrullinated versus uncitrullinated GNS or FLNA. Plates were coated with GNS (A) or FLNA (C), with or without citrullination, incubated with serum from patients or HC subjects, and tested in duplicate. Symbols represent values for individual patients, and horizontal lines indicate the mean values. In A and C, only significant P values, calculated by an unpaired, 2-tailed t test with Welch’s correction, are shown. (B) Correlation between IgG antibody responses against citrullinated GNS or citrullinated FLNA (D) and ACPA levels in the 46 patients with RA. The r and P values shown in B and D were determined by Spearman’s correlations. citGNS, citrullinated GNS; citFLNA, citrullinated FLNA. Utility of GNS and FLNA autoantibody evaluation in the diagnosis of RA. In our patient cohort, 70 (69%) of the 101 NORA and CRA patients were seropositive for ACPAs and/or RF, which are standard, commercially available autoantibody determinations for support of the diagnosis of RA. Among the 31 patients who did not have a positive test for ACPAs and/or RF, 13 had a positive test for IgG and/or IgA GNS autoantibodies and 9 had a positive test for IgG and/or IgA FLNA autoantibodies. Taken together, 17 of the 31 seronegative patients (55%) had such autoantibodies, 15 of whom could be identified with the IgG test alone. Overall, when autoantibody responses against GNS and FLNA were combined with standard autoantibody determinations, 87 (86%) of the 101 patients with RA had a positive test result for support of the diagnosis, and only 14 (14%) lacked a specific marker for RA. GNS and FLNA protein levels in serum and joints. For a self-protein to become the target of autoimmune responses in RA patients’ inflamed joints, one would predict that the protein would be present at high concentrations there. For this purpose, we measured GNS and FLNA protein concentrations in serum samples from the 101 patients with RA and in synovial fluid (SF) from 17 patients for whom such samples were available. The levels of GNS were higher in the serum of RA patients than were GNS levels in the control groups (P ≤ 0.002) (Figure 5A), and in RA patients, the levels of this protein tended to be higher in SF than in serum. Similarly, FLNA protein levels were significantly higher in the serum of RA patients than in HCs (P < 0.0001), but in RA patients, FLNA protein levels in SF and serum were similar (Figure 5B). Figure 5 GNS and FLNA protein levels in RA patients and comparison group subjects. GNS and FLNA protein concentrations were measured in serum and SF samples from patients with RA, serum samples from patients with CTD, SpA, or LA, and serum samples from HCs. (A) GNS protein concentrations and (B) FLNA protein concentrations are shown, as measured by ELISA assay. For both analyses, positivity was defined as greater than 3 SD above the mean value for HC subjects (area above the shaded region). Symbols represent values for individual patients, and horizontal lines indicate the mean values. The values for patient RA1 are indicated with a star. Only significant P values, determined by unpaired, 2-tailed t test with Welch’s correction, are shown. SLE, systemic lupus erythematosus. To gain further insight into the protein abundance and distribution, synovial tissues from 10 patients, 4 with RA and 3 each with LA or osteoarthritis (OA), were stained for expression of GNS and FLNA, using immunohistologic methods. GNS showed a fine, reticular pattern in and around endothelial cells in 3 of the 4 patients with RA, but not in those with LA or OA (Figure 6). We observed that FLNA was intensely expressed in the tunica muscularis around blood vessels and in large or elongated cells, presumably synoviocytes, and it was also faintly expressed in the extracellular matrix. We detected FLNA expression in all RA patients, lesser staining in 2 of the LA patients, but no staining in the OA patients (Figure 6). Thus, in RA, these 2 proteins were present in inflamed synovial tissue, particularly around blood vessels, where they could become targets of autoimmune responses. Figure 6 Immunohistochemical staining of synovial tissue for GNS and FLNA. Representative synovial tissue images from 1 patient with RA, 1 with LA, and 1 with OA are shown for expression of GNS or FLNA protein. Brown color indicates specific staining of GNS or FLNA self-proteins, and purple indicates hematoxylin staining. Images were taken at ×20 magnification, and ×40 magnification was used for the RA patient to highlight the staining around blood vessels. Sequence homology between T cell epitopes of microbial and self-peptides. In an effort to determine whether T cell epitope mimicry may play a role in linking Prevotella reactivity with GNS and FLNA autoimmune responses, the sequence of each of the 2 self-peptides isolated from patient RA1 was used first to search for regions of similarity with any microbial protein using the microbial protein database in BLASTP (Basic Local Alignment Search Tool, protein) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). For both self-peptides, Prevotella spp. peptides were among the top sequences producing significant alignment, specifically in areas predicted to be in the HLA-DR–binding groove. Therefore, we refined the search for sequence similarity by screening only microbial sequences from Prevotellaceae (NCBI Entrez Genome taxid:171552). To evaluate sequence homology, self- and microbial peptides were aligned using the program Clustal Omega (28). The peptide derived from GNS had 67% sequence homology with a peptide from the Prevotella arylsulfatase protein (WP_062433009) (Figure 7A), which was predicted by CELLO software to have a periplasmic location (29). Importantly, the major area of homology for this microbial peptide was restricted to amino acids predicted to be in the HLA-DR–binding groove (Figure 7A). For the Prevotella peptide, 5 of the 9 amino acids were identical to one of the predicted binding registers (P1 = the first F) in the GNS peptide. Moreover, the peptides shared amino acid identity at the P1, P4, and P6 sites, which are critical for peptide binding, as well as in the flanking regions at each end of the peptide, which also influence peptide binding (Figure 7A). The FLNA peptide had 80% identity with a peptide derived from an uncharacterized Prevotella protein (WP_028897633) (Figure 7A), which was predicted to have an extracellular location (a secreted protein). Moreover, the major area of homology was again found in the HLA-DR–binding groove, where 7 of the 9 amino acids were identical in the Prevotella and FLNA peptides, and the remaining 2 amino acids had conserved properties (Figure 7A). Figure 7 Sequence homology between self- and microbial peptides. (A) Sequence alignment of the self- and corresponding microbial peptides is shown (Clustal Omega), and the predicted binding frames of the self-peptides are given for the HLA-DRB1*0101 and *0401 molecules. Red residues indicate the P1 position (TEPITOPE predicted 3 binding registers for GNS [both HLA-DRB1*0101 and *0401], 2 for FLNA [HLA-DRB1*0401], and 1 for FLNA [HLA-DR*0101]), and blue residues indicate positions P2 through P9. The line through the amino acid residues indicates that the peptide-binding register contains an amino acid with an R-group that may not interact favorably with one of the MHC-binding pockets. (B) PBMCs from 24 RA patients and 10 HCs were incubated with 1 of the 2 self-peptides (GNS or FLNA) or each of the 2 corresponding microbial peptides (1 μM each). In each assay, a positive control (phytohemagglutinin) and a negative control (no peptide) were included. The amount of IFN-γ secretion is shown, as determined by ELISpot assay. A positive response was defined as greater than 3 SD above the mean value for the HCs (area above the shaded region). Horizontal lines represent the mean values for each group. *P < 0.05 and **P < 0.005, by unpaired, 2-tailed t test with Welch’s correction. (C) Correlations between the T cell reactivity to the GNS peptide and the 2 corresponding microbial peptides, 1 derived from the Prevotella arylsulfatase protein and the other from the Parabacteroides GNS protein. (D) Correlations between the T cell reactivity to the FLNA peptide and the 2 corresponding microbial peptides derived from 2 hypothetical proteins, 1 from the Prevotella sp. and the other from the Butyricimonas sp. P and r values shown in C and D were calculated using Spearman’s correlation test. For comparison, we analyzed the GNS and FLNA sequences for homology with P. gingivalis, a periodontal pathogen of interest in RA, using Porphyromonadaceae (taxid:171551) as the reference database in the BLASTP search. However, we found no homology between P. gingivalis and GNS or FLNA sequences. P. gingivalis also stimulates antibody responses in the subgroup of RA patients who have periodontal disease (27, 30), but there is little overlap between RA patients with P. gingivalis antibodies and those with P. copri antibodies (25). Instead, among the Porphyromonadaceae, the GNS epitope had partial sequence similarity with a peptide from the periplasmic protein N-acetylgalactosamine-6-sulfatase of the Parabacteroides sp. (WP_046148720) (Figure 7A). Thus, as with the homology between the GNS peptide and the peptide from the Prevotella arylsulfatase protein, the Parabacteroides protein was also a sulfatase. These enzymes are key in the adaptation and persistence of human commensal bacteria in the gut (31, 32). Moreover, the Parabacteroides peptide had 4 amino acids identical to the GNS peptide and 1 amino acid with conserved properties. These included amino acids with shared identity in the P1 through P4 and P6 sites. A similar evaluation of the FLNA peptide showed sequence homology with a predicted cytoplasmic uncharacterized protein of the Butyricimonas sp. (WP_065219401.1), another gut commensal. The Butyricimonas peptide shared identity with 6 of 9 amino acids in one of the predicted HLA-DR registers (P1 = F) of the FLNA peptide, and two of the three remaining amino acids had conserved properties (Figure 7A). Prevotella, Parabacteroides, and Butyricimonas are each members of the Bacteroidetes phylum, one of the two major phyla of gut commensal organisms. T cell responses to homologous microbial and self-peptides. To address whether patients had reactivity to these self-epitopes and the corresponding microbial epitopes, we performed ELISpot assays with each of these peptides using PBMCs from the 24 patients with NORA in whom sufficient numbers of cells remained and from 10 HCs. When cells were stimulated with the GNS peptide or each of the 2 corresponding microbial peptides (1 derived from Prevotella and the other from Parabacteroides), we found Th1 cell reactivity to all 3 peptides. Of the 24 RA patients, 8 (33%) had T cell reactivity to the GNS peptide, 9 (38%) showed responses to the Prevotella peptide, and 6 (25%) had reactivity to the Parabacteroides peptide, all of which were responses that were 3 SD or more above the mean values for HCs (Figure 7B). Of the 8 patients who had reactivity to the GNS peptide, 7 also had responses to the microbial peptides. When PBMCs were incubated with the FLNA peptide, 9 of the 24 patients with RA (38%) had T cell responses, 10 (42%) showed reactivity to the corresponding Prevotella peptide, and 7 (29%) had responses to the Butyricimonas peptide (Figure 7B). Furthermore, all 9 patients with reactivity to the FLNA peptide also had responses to the microbial peptides. Thus, except for 1 patient, the same patients who had reactivity to the GNS and/or FLNA peptides also had responses to the corresponding microbial peptides. Additionally, among the microbial peptides, we observed a trend toward a higher percentage of patients who had reactivity to the Prevotella peptides than to the other gut commensals. Moreover, when the magnitude of the T cell responses to each self-peptide was correlated with that of the corresponding microbial peptides, the responses to the GNS or FLNA peptide strongly correlated with reactivity to each of the 2 microbial peptides (in each instance, P < 0.0001) (Figure 7, C and D). Therefore, the stronger the response to the microbial peptides, the greater the response to the self-peptide. In contrast, PBMCs from 10 HC subjects did not show a correlation with any of the self- or microbial peptides (Figure 7, C and D). Of the 24 RA patients tested with the single peptides of GNS and FLNA (Figure 7), 17 were initially analyzed for T cell reactivity to pools of 4 peptides derived from these proteins (Figure 1). Only 4 of the 17 patients responded to the pool of peptides and not to the single peptide, suggesting that the majority of patients had reactivity to the single peptide epitope. Only 2 of the 17 patients responded to the single peptides, but failed to respond to the peptide pools. Using an in silico prediction method (Immune Epitope Database [IEDB] Analysis Resource tool; http://tools.immuneepitope.org/mhcii/), the GNS peptide and the corresponding microbial peptides were predicted to bind HLA-DR molecules encoded by SE alleles with significantly higher affinity than non-SE alleles (Figure 8A). In addition, there was a trend toward greater affinity of SE binding of the FLNA peptide and the corresponding microbial peptides (Figure 8B). Of the 24 patients with RA, 15 (62%) had SE alleles. Consistent with the IEDB binding predictions, 9 of 11 patients (82%) with self- and microbial T cell reactivity had SE alleles compared with 5 of 13 patients (38%) without T cell responses to these antigens (P = 0.05). Thus, patients who had reactivity to the self-peptides often responded to the corresponding microbial peptides; the magnitude of the self-responses showed a significant correlation with the microbial responses, and these were more frequent in patients with SE alleles. Figure 8 HLA-DR–binding prediction for self- and microbial peptides. Prediction analyses were performed using the IEDB Analysis Resource consensus tool. A low percentile rank indicates good peptide binders. (A) MHC class II–binding prediction of the GNS peptide and the corresponding microbial peptides. (B) MHC class II–binding prediction of the FLNA peptide and the corresponding microbial peptides. SE alleles: *0101, *0102, *0401, *0404, and *1001. Non-SE alleles: *0103, *0301, *0403, *0803, *1101, *1201, *1302, *1501, and *1601. Data represent median values with interquartile ranges. **P < 0.005, by Mann-Whitney U test. Discussion RA is an HLA class II–associated autoimmune disease in which mucosal immunity, often resulting from interaction with oral or gut microbes or from inhaled antigens in the lung, is hypothesized to cause autoimmune phenomena leading to joint inflammation and damage. However, the factors linking mucosal immunity to autoimmunity in joints have been unclear. In this study in which HLA-DR–presented peptides were identified directly from patients’ synovial tissue or PBMCs, 2 previously unidentified self-antigens, GNS and FLNA, were shown to be targets of T and B cell responses in 52% and 56% of RA patients, respectively. Importantly, the GNS and FLNA HLA-DR–presented T cell epitopes have considerable sequence homology with Prevotella epitopes and with similar epitopes from several related gut commensals belonging to the same order, particularly in areas predicted to be in the HLA-DR–binding groove. Moreover, T cell responses to the corresponding microbial and self-peptides were strongly correlated, suggesting that T cell epitope mimicry may provide a potential link between mucosal immunity and immune responses in affected joints. Moreover, GNS and FLNA autoantibodies correlated with P. copri antibody responses. This finding might also be due to cross-reactive Prevotella and host protein B cell epitopes, but we currently lack the recombinant microbial proteins to test this hypothesis directly. Alternatively, this correlation might simply be a reflection of T cell help resulting from T cell epitope mimicry. The presence of natural IgM, IgG, or IgA autoantibodies has been reported in the serum of normal control subjects and is characterized by broad reactivity to self- and microbial antigens (33, 34). However, our findings cannot be explained simply by nonspecific physiological responses. First, GNS and FLNA autoantibodies were increased specifically in a large subgroup of patients with RA, and not in patients with other rheumatic diseases or in HCs. Second, these self-autoantibodies correlated strongly with P. copri antibodies, but not with P. gingivalis antibodies. Third, patients with RA, but not healthy subjects, often had both T and B cell responses to these self-antigens. For control of the microbiota, the generation of specific, high-affinity IgA antibody responses requires T and B cell interactions (35), whereas the “natural” antibody pool consists primarily of low-affinity polyreactive IgA antibodies, whose production is independent of T cell help (36–38). Currently, the only known antibody responses that are specific for RA are directed against citrullinated proteins, in particular against enolase, vimentin, and fibronectin (39). Moreover, there is hypercitrullination of a range of proteins in the joints of many patients with RA (40). In our study, the GNS protein appeared to be citrullinated in vivo, and antibodies against it correlated with ACPA levels, whereas levels of antibodies against the FLNA protein, which did not appear to be citrullinated, did not correlate with ACPA levels. Moreover, HLA-DR molecules encoded by RA SE alleles bound the GNS peptide and, to a lesser degree, the FLNA peptide with higher affinity than did non-SE alleles, and SE alleles largely influence the generation of ACPAs (3). Both FLNA and GNS self-antigens, which are often highly expressed in RA synovial tissue, represent good autoimmune targets. GNS is an enzyme located in lysosomes and is involved in the degradation and recycling of different molecules, such as glycosaminoglycans (GAGs) (https://ghr.nlm.nih.gov/gene/GNS), a major component of joint cartilage and other soft connective tissues (41). FLNA is a ubiquitous, fundamental protein for building the cell cytoskeleton and organizing the extracellular matrix (42). In addition, FLNA is required for cell-cell contact in vascular development (43). Consistent with its function, we found that FLNA protein was especially prominent in the tunica muscularis around blood vessels in RA synovial tissue and was also faintly expressed in the extracellular matrix. However, it is not yet clear why the GNS protein was seen only in a reticular pattern around blood vessels. Nevertheless, the important point is that both host proteins would be available for HLA-DR presentation in synovial tissue and both represent potential targets for autoimmune responses. High-throughput sequencing of stool samples in 2 studies of RA patients showed gut dysbiosis (13, 14), and 1 study reported overexpansion of Prevotella spp., particularly P. copri (13). However, little is known about potential bowel pathology in RA. The major limitation of our study is the lack of specific information about pathology or immune responses in the bowel. Our study of patients with NORA was originally intended as a study of mouth flora and periodontal disease (30), and, therefore, the collection of stool samples was not a part of our protocol. Nevertheless, the finding of gut dysbiosis favoring Prevotella (13), the identification of P. copri as an immune-relevant bacterium in RA (25), and the finding here of specific T and B cell responses to Prevotella and corresponding GNS and FLNA epitopes suggest that mucosal immune responses in the gut may be a part of the disease in a sizable subgroup of RA patients. Although DMARD therapy may resolve dysbiosis (14) and P. copri overexpansion (13), immune reactivity to the organism, once triggered, appeared to persist or even increase in patients with CRA who were taking these medications. In contrast, patients with SpA or postinfectious, antibiotic-refractory LA, which may also be treated with DMARDs, did not have reactivity to this organism (25). Taken together, our studies highlight a possible mechanism linking gut and joint inflammation. We hypothesize that dysbiosis or low-grade gut inflammation may compromise the mucosal barrier and result in leakage of commensal organisms, leading to activation of lymphocytes targeting microbial antigens. Here, we provide evidence that T cell epitopes of a related order of gut microbes, particularly Prevotella spp., may cross-react with self-epitopes of highly expressed proteins in joints, especially in patients with SE alleles. T cells activated by microbial peptides at the mucosal surface may then home to inflamed joints and cross-react with homologous self-antigens. In addition, as we have previously reported (25), commensal organisms or their remnants or microbe-activated antigen-presenting cells (APCs) may occasionally home to joints, where they further amplify joint inflammation and restimulate self-reactive T cells. Thus, an initial trigger in the gut mucosa may activate cross-reactive microbial immune responses that have the potential to shift to an autoimmune phenotype at sites where the homologous self-antigens are expressed. Even though proof of cross-reactivity will require the cloning of single T cells, the prominence of homologous epitopes among gut microbes and epitopes from 2 self-proteins in synovial tissue, the greater affinity of HLA-DR molecules encoded by SE alleles in binding these epitopes, and the demonstration that RA patients often have T cell responses to corresponding microbial and self-epitopes suggest the potential for these immune responses to have a role in RA pathogenesis. We do not think that these mechanisms are the entire explanation for the marked inflammatory and proliferative responses in the RA synovial lesion. Rather, we propose that these mechanisms play a role in linking gut and joint immune responses and in amplifying synovial inflammation in a large subgroup of RA patients. Although knowledge of bowel pathology is not yet clear in patients with RA, the identification of immune responses to P. copri, GNS, and FLNA has practical implications for the diagnosis and treatment of the disease. First, these immune responses appear to be specific for RA. As shown here, the addition of GNS and FLNA to standard autoantibody determinations (ACPAs and RF) increased the percentage of seropositive patients from 69% to 86%, thereby improving the diagnostic support for some patients with seronegative RA, who currently lack specific markers. Furthermore, the inclusion of citrullinated GNS in a test for ACPAs may increase the sensitivity of that test. These determinations have increased in importance, because earlier treatment with DMARDs may improve long-term outcomes, whereas a lack of diagnostic markers for seronegative patients may delay appropriate DMARD treatment, leading to less favorable clinical outcomes (44, 45). Finally, in addition to DMARDs, the identification of patients with these antibody responses may allow the testing and development of adjunctive forms of treatment, such as brief, targeted antibiotic regimens or diet alterations. Ultimately, the identification of pathogenic T cell epitopes in synovial tissue, as was done here, may make it possible to engineer blocking peptides, which would limit autoimmune stimulation and ameliorate these presumably disadvantageous autoimmune responses. Methods Patients and control subjects. The 101 patients in this study met the American College of Rheumatology and the European League Against Rheumatism Collaborative Initiative criteria for the diagnosis of RA (46). Of these 101 patients, 49 had NORA for 12 months or less and had not yet been treated with DMARDs. The remaining 52 patients had CRA of more than 1 year’s duration (usually for many years), often treated with DMARDs. For isolation of HLA-DR–presented peptides, synovial tissue and PBMCs were obtained from patient RA1, who was undergoing arthroscopic synovectomy. To test the implicated peptides and their source proteins for immunoreactivity in additional patients, PBMCs and serum samples were collected from patients with NORA, but only serum samples were available from patients with CRA. For other comparison groups, serum samples were collected from 28 patients with CTD, including systemic lupus, mixed CTD, scleroderma, and Sjögren’s syndrome; 28 patients with SpA; and 50 patients with LA. Additionally, serum samples and PBMCs were collected from 15 healthy hospital personnel who did not have a history of RA or other autoimmune diseases, and serum samples were obtained from 40 healthy blood bank donors. HLA-DR typing was performed on blood samples from all RA or LA patients and from healthy subjects at the American Red Cross Laboratory in Dedham, Massachusetts, USA. ELISpot T cell assay. A detailed description of the methods for the isolation and identification of HLA-DR–presented peptides is given in our previous publications (19, 20). In the current study, all nonredundant HLA-DR–presented peptides identified from patient RA1 were synthesized (Mimotopes) and tested for reactivity in sets of 3 (1 μM of each peptide) using the patient’s PBMCs in IFN-γ ELISpot assays. In this initial screen, 2 self-peptides derived from GNS and FLNA were implicated; the remaining 138 peptides showed no reactivity. For testing in larger numbers of patients and control subjects, who would have a range of HLA-DR genotypes, the original peptides identified from the patient (shown in bold) and 3 additional promiscuous peptides that were predicted to bind 16 or more HLA-DR molecules were synthesized and HPLC purified at the MGH Core Facility. The GNS peptide sequences were as follows: 47PNVVLLLTDDQDE59, 222FEPFFMMIATPAPH235, 451TYACVRTMSALWNLQ465, 504NYRLMMLQSCSGPTC518. The FLNA peptide sequences were as follows: 73DGLRLIALLEVLSQKK88, 118SIKLVSIDSKAIVDG132, 416VEVVIQDPMGQKG428, 2446NPAEFVVNTSNAGAG2460. To assess microbial sequence homology with the GNS peptide 222FEPFFMMIATPAPH235, the following microbial peptides were tested: 231KKPFFMMVAMNPPH245, which was derived from the arylsulfatase protein of the Prevotella spp. (WP_062433009.1) and 247DVPFFMMWTTPLPH261, derived from the GNS of the Parabacteroides sp. (WP_046148720.1). To evaluate the microbial sequence homology for the FLNA peptide 2446NPAEFVVNTSNAGAG2460, the following microbial peptides were tested: 133QGFVVKTANAGAL146, which was derived from an uncharacterized protein of the Prevotella sp. (WP_028897633.1) and 90ANFMINVSNAGAL103, derived from an uncharacterized protein of the Butyricimonas sp. (WP_065219401.1). The RA patients’ PBMCs were stimulated with the pool of peptides or single peptides and analyzed for reactivity to a human IFN-γ/IL-17 Double-Color ELISpot Kit (Cellular Technology Ltd.). All peptides (1 μM) were tested in duplicate wells, as were positive (phytohemagglutinin) and negative (no antigen) control samples. After 5 days, cells were transferred to ELISpot plates coated with IFN-γ/IL-17 antibodies and incubated overnight. Images of wells were captured using an ImmunoSpot Series 3B Analyzer (Cellular Technology Ltd.), and spots were counted using ImmunoSpot software. A positive T cell response was defined as 3 SD above the mean value for HC subjects. ELISA for serum IgG and IgA anti-GNS and anti-FLNA autoantibodies. ELISA plates were coated with 0.5 μg/ml rhGNS (Novoprotein) or rhFLNA (Novusbio) overnight at 4°C. Subsequent incubations and washes were performed at room temperature. After washing with PBS containing 0.05% Tween-20 (PBST), the plates were blocked with blocking buffer (5% nonfat dry milk in PBST) for 1 hour. Afterwards, 100 μl of each patient’s serum sample (diluted 50-fold) was added in duplicate wells for 1.5 hours, followed by HRP-conjugated goat anti-human IgG (sc-2453; Santa Cruz Biotechnology Inc.) or HRP-conjugated goat anti-human IgA (STAR141P; Bio-Rad), and then tetramethylbenzidine (TMB) substrate (BD). For interplate standardization, 2 control samples were included with each assay. In vitro citrullination assay. rhGNS (2 μg) or rhFLNA (2 μg) was incubated with rhPAD4 (400 ng), which was provided by Maximilian Koenig from the laboratory of Felipe Andrade (Johns Hopkins School of Medicine, Baltimore, Maryland, USA) in 1 M Tris (pH 7.6) in the presence of 200 mM CaCl2. A negative control reaction was performed, substituting 200 mM CaCl2 with 200 mM EDTA. Incubation was performed at 37°C for 3 hours. Protein citrullination was determined by anti–modified citrulline immunoblotting, according to the manufacturer’s recommendations (EMD Millipore). Quantification of GNS and FLNA protein levels in serum and joint fluid. ELISA plates were coated with 5 μg/ml capture antibody (sc-161669 for GNS and sc-58764 for FLNA; Santa Cruz Biotechnology Inc.) overnight at 4°C. Plates were then washed with PBS and blocked with PBST containing 5% milk (blocking buffer) for 1 hour. Afterwards, serum or joint fluid samples (diluted 1:10 in blocking buffer) were added in duplicate and incubated for 2 hours at room temperature. Next, a detection antibody (5 μg/ml) (SAB1410557 for GNS from Sigma-Aldrich; sc-28284 for FLNA from Santa Cruz Biotechnology Inc.) was added for 2 hours. The plates were then incubated with goat anti-rabbit IgG-HRP (sc-2030; Santa Cruz Biotechnology Inc.), followed by TMB substrate. For interplate standardization, 2 control samples were included on each plate. Immunohistochemistry. Fresh-frozen synovial tissue samples were stained for GNS and FLNA proteins. After blocking, the sections were incubated with a rabbit polyclonal antibody against GNS (SAB1410557; Sigma-Aldrich) and a mouse monoclonal antibody against FLNA (sc-17749; Santa Cruz Biotechnology Inc.) at 4°C overnight. For negative controls, nonspecific rabbit or mouse IgG antibodies (Sigma-Aldrich) were used. The following day, the sections were incubated with a biotinylated anti-rabbit (HK336-5R; Biogenex) or anti-mouse (HK335-5M; Biogenex) secondary antibody, peroxidase-streptavidin, and then a diaminobenzidine substrate. The slides were counterstained with Mayer’s hematoxylin and mounted with Permount Mounting Medium (Fisher Scientific). Microscopic images were obtained with a Zeiss widefield microscope. In silico determinations of HLA-DR–binding affinity. The affinity of HLA-DR binding of the GNS, FLNA, and related microbial peptides was determined using the T cell epitope – MHC class II molecules binding prediction tool from the IEDB Analysis Resource. This tool uses different methods to predict MHC class II epitopes, including a consensus approach that combines NN-align, SMM-align, and combinatorial library methods. For the analysis, all HLA-DR alleles found in the RA cohort were analyzed, including 5 SE alleles and 9 non-SE alleles. Statistics. Categorical data were analyzed by Fisher’s exact test, and quantitative data were analyzed using an unpaired, 2-tailed t test with Welch’s correction. Correlations were determined using Spearman’s correlation test. All analyses were performed using GraphPad Prism 6 (GraphPad Software). All P values are 2-tailed. P values of 0.05 or less were considered statistically significant. Study approval. The present study was reviewed and approved by the Human Investigations Committee at MGH (2008 to 2014). All patients and control subjects provided written informed consent prior to their participation in the study. Author contributions AP designed and performed the experiments and analyzed the data. SLA and ACS enrolled and cared for patients in the study, handled clinical data collection, and performed clinical correlations. QW and CEC performed mass spectrometric analyses. EED, KS, and ACS provided advice and data analysis interpretation. All authors contributed to the preparation of the manuscript. Acknowledgments We thank Deborah Collier and Marcy Bolster (MGH, Boston, MA) for help with patient care; Maximilian Koenig and Felipe Andrade (Johns Hopkins University, Baltimore, MD) for providing the human PAD4 enzyme for citrullination assays; Gunnlaugur Petur Nielsen (MGH, Boston, MA) for help with the description of synovial immunohistology; Dennis Burke (MGH, Boston, MA) and John Aversa (Yale University, New Haven, CT) for help with obtaining synovial tissue; Mandakolathur Murali (MGH, Boston, MA) for RF and ACPA analyses; the American Red Cross laboratory for HLA-DR typing of patients; and Katherine Sulka (MGH, Boston, MA) for help with the preparation of samples for HLA-DR typing. This work was supported by grants from the American College of Rheumatology Innovative Grant Program “Within our Reach, Finding a Cure for RA;” the Ounsworth-Fitzgerald Foundation; the Mathers Foundation; the English, Bonter, Mitchell Foundation; the Littauer Foundation; the Lillian B. Davey Foundation; the Eshe Fund (to ACS); and the NIH (P41 GM104603, S10 RR020946, and S10 OD010724, to CEC). SLA received support from a Scientist Development Award from the Rheumatology Research Foundation. Footnotes EED’s present address is: Agenus Inc, Lexington, Massachusetts, USA. QW’s present address is: Waters Corporation, Milford, Massachusetts, USA. Conflict of interest: The authors have declared that no conflict of interest exists. Reference information: J Clin Invest. 2017;127(8):2946–2956.https://doi.org/10.1172/JCI93450. References Li Y, et al. Deficient activity of the nuclease MRE11A induces T cell aging and promotes arthritogenic effector functions in patients with rheumatoid arthritis. Immunity. 2016;45(4):903–916. View this article via: PubMed CrossRef Google Scholar Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30(11):1205–1213. View this article via: PubMed CrossRef Google Scholar McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. View this article via: PubMed CrossRef Google Scholar Nishimura K, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146(11):797–808. View this article via: PubMed CrossRef Google Scholar Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101(1):273–281. View this article via: JCI PubMed CrossRef Google Scholar Suzuki K, et al. High diagnostic performance of ELISA detection of antibodies to citrullinated antigens in rheumatoid arthritis. Scand J Rheumatol. 2003;32(4):197–204. View this article via: PubMed CrossRef Google Scholar del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum. 1988;31(10):1239–1244. View this article via: PubMed CrossRef Google Scholar Majka DS, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67(6):801–807. View this article via: PubMed CrossRef Google Scholar Nielen MM, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–386. View this article via: PubMed CrossRef Google Scholar Bachmaier K, Neu N, de la Maza LM, Pal S, Hessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283(5406):1335–1339. View this article via: PubMed CrossRef Google Scholar Miller SD, et al. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3(10):1133–1136. View this article via: PubMed CrossRef Google Scholar Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279(5355):1344–1347. View this article via: PubMed CrossRef Google Scholar Scher JU, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. View this article via: PubMed Google Scholar Zhang X, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895–905. View this article via: PubMed CrossRef Google Scholar Maeda Y, et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016;68(11):2646–2661. View this article via: PubMed CrossRef Google Scholar Hemmer B, et al. Predictable TCR antigen recognition based on peptide scans leads to the identification of agonist ligands with no sequence homology. J Immunol. 1998;160(8):3631–3636. View this article via: PubMed Google Scholar Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4(7):781–785. View this article via: PubMed CrossRef Google Scholar Ehl S, et al. Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. J Exp Med. 1998;187(5):763–774. View this article via: PubMed CrossRef Google Scholar Seward RJ, Drouin EE, Steere AC, Costello CE. Peptides presented by HLA-DR molecules in synovia of patients with rheumatoid arthritis or antibiotic-refractory Lyme arthritis. Mol Cell Proteomics. 2011;10(3):M110.002477. View this article via: PubMed CrossRef Google Scholar Wang Q, et al. Immunogenic HLA-DR-presented self-peptides identified directly from clinical samples of synovial tissue, synovial fluid, or peripheral blood in patients with rheumatoid arthritis or Lyme arthritis. J Proteome Res. 2017;16(1):122–136. View this article via: PubMed CrossRef Google Scholar Drouin EE, et al. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2013;65(1):186–196. View this article via: PubMed CrossRef Google Scholar Crowley JT, et al. A highly expressed human protein, apolipoprotein B-100, serves as an autoantigen in a subgroup of patients with Lyme disease. J Infect Dis. 2015;212(11):1841–1850. View this article via: PubMed CrossRef Google Scholar Crowley JT, et al. Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J Autoimmun. 2016;69:24–37. View this article via: PubMed CrossRef Google Scholar Pianta A, et al. Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin Immunol. 2015;160(2):336–341. View this article via: PubMed CrossRef Google Scholar Pianta A, et al. Evidence for immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69(5):964–975. View this article via: PubMed CrossRef Google Scholar Sturniolo T, et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 1999;17(6):555–561. View this article via: PubMed CrossRef Google Scholar Mikuls TR, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(5):1090–1100. View this article via: PubMed CrossRef Google Scholar Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. View this article via: PubMed Google Scholar Yu CS, Lin CJ, Hwang JK. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 2004;13(5):1402–1406. View this article via: PubMed CrossRef Google Scholar Arvikar SL, et al. Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R109. View this article via: PubMed CrossRef Google Scholar Benjdia A, Martens EC, Gordon JI, Berteau O. Sulfatases and a radical S-adenosyl-L-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron. J Biol Chem. 2011;286(29):25973–25982. View this article via: PubMed CrossRef Google Scholar Ulmer JE, et al. Characterization of glycosaminoglycan (GAG) sulfatases from the human gut symbiont Bacteroides thetaiotaomicron reveals the first GAG-specific bacterial endosulfatase. J Biol Chem. 2014;289(35):24289–24303. View this article via: PubMed CrossRef Google Scholar Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7(6):812–818. View this article via: PubMed CrossRef Google Scholar Dighiero G. Natural autoantibodies, tolerance, and autoimmunity. Ann N Y Acad Sci. 1997;815:182–192. View this article via: PubMed CrossRef Google Scholar Kroese FG, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1(1):75–84. View this article via: PubMed CrossRef Google Scholar Macpherson AJ, Gatto D, Sainsbury E,

|
Scooped by
Gilbert C FAURE
January 18, 2021 12:37 PM
|
T regulatory cells (Tregs) plays an important role in maintaining self-tolerance and preventing autoimmune diseases by inhibiting proliferation and cytokine production of self-reactive T cells.Controversy was reported regarding the frequency of CD4+CD25+ Tregs in the peripheral circulation of rheum...

|
Scooped by
Gilbert C FAURE
November 12, 2020 3:55 AM
|
BACK TO SEARCH RESULTS Low Back Pain William Oswald, PT, DPT, OCS CE Information Downloads Course Brochure Contains CE information for additional professionals LIVE ONLINE VIDEO ONLINE TEXT Course Highlights Relevant and most recent low back pain research Practical assessment and treatment strategies you can use the next day Effective, evidence-based interventions for the management and treatment of the most common diagnosis in out-patient rehabilitation Additional Info Description Learning Objectives Outline Instructor Bio & Disclosures CE Credits FAQs Back pain is the most common reason people seekhealth care. Research has revealed the high risk and small rewards from opioid drugs and surgery, and patients need new viable, conservative treatment options. The art andscience of medicine appear in conflict, forcing clinicians to choose between the sides of experience and evidence. In this course, the balance of art and science is exploredcombined with extensive clinical experience to highlight examination and treatment strategies that are both efficientand effective in treating low back pain. Compare and contrast the differences inpathoanatomical and the biopsychosocial models of low back care. Choose the best screening tests for systemic disease and non-musculoskeletal pathology in patients with low back pain. Identify subgroups of patients that would benefit frommanual therapy or integrated exercise through clinical reasoning and application of current best evidence. Introduction and Screening Current research and practice guidelines Risk and prognostic indicators Imaging and diagnostic triage Examination and Biopsychosocial Factors An expert interview Anxiety/depression/fear avoidance Objective exam Management Strategies Mobility Stability Chronic pain Other Spinal stenosis Sacroiliac joint dysfunction William Oswald, PT, DPT, OCS is a licensed physical therapist and a board certified orthopedic clinical specialist. Dr. Oswald has 20 years of extensive clinical experience in an outpatient orthopedic setting. His area of specialty is low back pain as a diplomat in Mechanical Diagnosis and Therapy. Dr. Oswald is a clinical manager at NYU Langone Health. He has co-authored research articles in the Journal of Orthopedic and Sports Physical Therapy, the Pain Journal, and the Journal of Manual & Manipulative Therapy. Dr. Oswald earned both a Master's and Doctorate degree in physical therapy from New York Institute of Technology. DISCLOSURES FINANCIAL: Bill Oswald is compensated by Summit as an instructor. NONFINANCIAL: Bill Oswald has no non-financial relationships to disclose. Search your profession and state above to see CE approval for your selected course. Content Level: Intermediate SATISFACTORY COMPLETION: Participants must pay tuition fee, sign in, attend the entire seminar, complete an evaluation and sign out in order to receive a certificate of completion. Participants not fulfilling these requirements will not receive a certificate. Failure to sign in or out will result in forfeiture of credit for the entire workshop. No exceptions will be made. Partial credit is not available. Scope of Practice: Workshop content is not intended for use by participants outside of the regulatory scope of practice of their license(s). You are responsible for knowing what lies within and without your professional scope of practice. Online Workshops: Why Summit? As a national leader in providing high quality live workshops for over a decade, Summit understands that your time and money are extremely valuable -- that you don't want to settle for Online Continuing Education that is poor quality, poorly delivered, or won't be accepted by state and national licensing boards. With that as our foundation, we are proud to now offer Online Continuing Education courses that we believe set a new standard of high quality. Leveraging our exceptional teaching faculty that delivers thousands of live workshops each year (with an average customer satisfaction rating of 4.7 out of 5.0), we have now invested in creating the same exceptional Online course experience. We deliver Online courses that are both high quality and easy to use: We invest in high quality video production Excellent sound, multiple cameras and camera angles "You are there" tight coverage of lab exercises Edited video content to focus on highest learning content Our Online platform is state of the art You can see video of the instructor and slides at the same time, plus also follow the course outline and download manual You can stop, start, & pick up again at your convenience (up to 30 days) We are mobile friendly, and also provide live telephone technical support, Monday - Friday 6AM to 6PM CST Finally, we are so confident that you will like our Online courses, we provide a 100% satisfaction guarantee. Watch up to the first 30 minutes, and if you are not satisfied, you will receive a full refund. Must contact Summit within initial 30-day viewing period. On-Demand: What to Expect We want to make sure that you are 100% comfortable with what you are purchasing, so this is a rundown of the experience you should expect. Here's what you can expect from our media player: Watch high quality video of your instructor while also viewing the power point presentation Compatibility with both Mac and PC Mobile compatibility so you can watch on your phone or tablet We have also built in multiple helpful links within the media player so you can: Download the manual Get technical support FAQs to common questions Launch your post test and obtain your certification Media Player Details This is what the media player will look like when you first launch the video: Note: The video will automatically start playing once you open the player. It may be beneficial to pause the video at the beginning so you can get organized and optimize your experience. Icons at the bottom You will notice 4 Icons on the bottom of the player, this is what these tabs do: Helpful details regarding the course, anything you need before starting, and how the video player works This is where you can see the course outline and download your manual FAQs on the most common questions to enhance your video experience Where your post course test and certification will be located after you finish the video Links in the top right hand corner There are also 3 links in the upper right hand corner: Support - Gives you the number to the technical support team for issues with the media player Account - Takes you to your online profile, and is also where you log out of the media player Logout - Takes you back to the Summit Professional Education website Continuing Education Credit FAQs How do I know if the workshop is approved for CE credit for my profession and state? We do the work upfront and when you select your profession and state, we only present courses that have CE approval for that profession and state. You can also view the licensing approval by profession on the Product page of any course (middle of page, far right tab of "CE Credit") If you have further questions regarding your CE approval, please contact Summit Customer Service M-F from 6 a.m. to 6 p.m. CST at (800) 433-9570 or email ceinfo@summit-education.com. Why aren't all online workshops approved for my profession and state? The approval of online workshops varies by state and profession, and some licensing boards have longer approval turnarounds than others. How many credits do I receive for an online course? Typically you will receive the same number of credits that you would get at a live workshop. Currently, all of our online workshops range from 1-6 credit hours (with some exceptions based on state and profession). You can view the licensing approval by profession on the Product page (middle of page, far right tab of "CE Credit") where you can see the exact number of credit hours for each profession and state. If you have further questions regarding CE credits, please contact Summit Customer Service M-F from 6 a.m. to 6 p.m. CST at (800) 433-9570 or email customerservice@summit-education.com. Does Summit report to CE broker? ASHA? Yes. Once you complete your online course, post-course requirements, and the printing of your certificate, please follow the below steps. For ASHA reporting an online course: Once you have met satisfactory completion requirements of the course, you will have access to a participant form via a "Report to ASHA" button in your account (next to the "Certificate Download" button). Simply select the "Report to ASHA" button, provide your ASHA member ID, and select "Submit". Please note, the "Report to ASHA" button will only be available in your account to submit your request for 10 days from the date you completed the online course after which Summit will be unable to report your completion to ASHA. Online course completions are reported to ASHA following the below schedule: Completions dated 1/1 - 3/31 will be reported to ASHA no later than 5/15 Completions dated 4/1 - 6/30 will be reported to ASHA no later than 8/14 Completions dated 7/1 - 9/30 will be reported to ASHA no later than 11/14 Completions dated 10/1 - 12/31 will be reported to ASHA no later than 2/14 Summit offers CE Broker reporting on behalf of FL PTs, OTs, SLPs and ATCs as well as for AL PTs, OH SLPs, SC PTs, OTs and SLPs. Licensees in states/professions outside of these listed should self-report at cebroker.com using the information provided on the certificate of completion. More information can be found here. Online Course Content FAQs Is an outline of the course content available before or during viewing? You can see a course outline on the Product page under the Course Outline tab. After purchase, the course outline is also provided on the media player for watching the course. How do I access the course manual? Once you have purchased the course, there is a tab at the bottom of the media player where you can download the manual to save or print for your personal use. Manual is located in the "Resources" tab Are there labs in the course? What do I do if there are labs during the course? Most of our courses have hands-on lab content, varying from minimal to significant. By looking at the Course Outline tab on the Product page, you can see how intensive the labs are for each course. During the lab section, we have invested heavily in providing very strong video content (i.e. very tight camera angles) so you can learn along with the live attendees. Depending on the course, you may want to simulate the activities that are presented in the course (which will be noted in the "Before You Start" section of the media player). Where can I find who is teaching the course and his/her professional credentials? You can see the instructor and their credentials on the product page under the Instructor Info tab Online Video Experience How long is the course? Courses range in length from 1-6 hours. You can see the exact length of the course by visiting the course Product page. Will I need any special materials to take the course? After purchasing, the any special materials or specific advice is listed at the bottom of the "Course Info" tab. Can I fast-forward or rewind through the online course? Yes. For your convenience, you may fast-forward or rewind while watching the course. However, please note you will be required to certify you have watched the entire course to obtain CE credit. Are there breaks? Can I press pause while watching the course? There are no formal breaks in the video content. However, there is a pause feature to allow taking breaks at your own convenience. Can I complete the course over several days? How much time do I have to finish the course? Yes, you can always press pause or stop to take a break at your own convenience. Also, if you leave the course, please note where you left so you can pick up where you last finished. However, please remember you need to finish the course and pass your post course test within 30 days (non-subscribers) or before your subscription ends (subscribers). Is the online course interactive? Will I be able to ask questions during the course? The video that you will be watching is a recorded version of a live workshop. The video captures Instructor answers to participant questions, but there is no opportunity for you to ask questions with the instructor, real time. If you do have questions for the instructor, most instructors have their email address at the end of their presentations. Am I able to re-watch the online courses even after I have completed the post course test and received my certificate? Once you purchase the online workshop, you have 30 days to view the workshop as many times as you want as well as to review the manual (if you choose not to download while taking the course). For subscribers, your access is unlimited while your subscription is active. Can I get a refund if I don't like the course? We want your experience with Summit to be an enjoyable one. If you purchase an online workshop and don't like it for whatever reason, please stop within the first 30 minutes to receive a full refund. Then call us at (800) 433-9570 to talk with our customer service team to get your refund or a new online course. Test & Certificate Will I need to take a post course test? Why? Yes, you are required to successfully pass the post course test to obtain CE credits and certification. This is required by our licensing boards for online courses. What is the post course test like, what is a passing score, and how many times can I take it? Post course tests consist of True/False and/or multiple choice questions. You will be able to retake the post course test as many time as necessary to receive a passing grade. A passing "grade" is 75% or higher for general content, 80% or higher for certain state specific workshops, like ethics, jurisprudence, and laws and rules courses. You will be able to retake the post course test as many times as necessary to receive a passing grade. When will I receive my credits and certificate? Once you complete your course evaluation and online test, you will be able to immediately download your certificate and request CE Broker or ASHA Reporting (if applicable). Technical FAQs What browsers and Operating system do I need to run for this to work properly? The following are the supported browsers and Operating Systems for the media player to work properly: Internet Explorer 11 or Microsoft Edge 44+ for Windows 10 Google Chrome 75.0+ for Windows, Mac OSX, or Linux Mozilla Firefox 70.0+ for Windows, Mac OSX, or Linux Safari 12.0+ for Mac OSX 10.13 and above Will the media player work on both a Mac and PC? Yes, the online courses will play on both a Mac and PC. The following are the supported browsers and Operating Systems for the media player to work properly: Internet Explorer 11 or Microsoft Edge 44+ for Windows 10 Google Chrome 75.0+ for Windows, Mac OSX, or Linux Mozilla Firefox 70.0+ for Windows, Mac OSX, or Linux Safari 12.0+ for Mac OSX 10.13 and above Can the workshops be played on my phone? Yes! You are able to watch the workshops on your phone. Here are the technical requirements to ensure it works properly: Safari on iOS 13+ Safari on iPadOS 13+ Chrome on Android 9.0+ Any running Android 9.0+ Are the videos downloadable or streaming? All of the videos stream from the media player. How does the streaming video work? Live streaming video is content sent in compressed form over the Internet and displayed by the viewer in real time. With streaming video or streaming media, a Web user does not have to wait to download a file to play it. Instead, the media is sent in a continuous stream of data and is played as it arrives. Will I experience any occasional buffering or freezing of the video? Because of our streaming video approach, this rarely happens. If it does, it's typically because of customer internet access quality issues and we will provide troubleshooting tips as well as telephone support from our help desk. Do you provide technical support? Our help desk is available to assist you by phone Monday through Friday, 6AM to 6PM at (800) 433-9570. Facility Details & Map Front Desk Phone: There may be a fee for parking on-property at this facility. Summit does not reimburse for venue parking fees. Alternative free and paid parking options may be available, please contact the venue for details. Reviews Summit Subscription "All Access" Unlimited Live & Online CE 1000s of Live Courses 350+ Online Courses One full year of CE One low price Only $299.99 Subscribe Now OR Buy this course for $Loading Add to Cart

|
Scooped by
Gilbert C FAURE
June 30, 2020 1:20 PM
|
The cytokine, interleukin-23 (IL-23), can be critical for the progression of inflammatory diseases, including arthritis, and is often associated with T lymphocyte biology. We previously showed that certain lymphocyte-independent, inflammatory arthritis and pain models have a similar requirement for tumour necrosis factor (TNF), granulocyte macrophage-colony stimulating factor (GM-CSF), and C-C motif ligand 17 (CCL17). Given this correlation in cytokine requirements, we explored whether IL-23 might interact with this cytokine cluster in the control of arthritic and inflammatory pain. The role of IL-23 in the development of pain-like behaviour was investigated using mouse arthritis models (zymosan-induced arthritis and GM-CSF-, TNF-, and CCL17-driven monoarticular arthritis) and inflammatory pain models (intraplantar zymosan, GM-CSF, TNF, and CCL17). Additionally, IL-23-induced inflammatory pain was measured in GM-CSF−/−, Tnf−/−, and Ccl17E/E mice and in the presence of indomethacin. Pain-like behaviour and arthritis were assessed by relative weight distribution in hindlimbs and histology, respectively. Cytokine mRNA expression in knees and paw skin was analysed by quantitative PCR. Blood and synovial cell populations were analysed by flow cytometry. We report, using Il23p19−/− mice, that innate immune (zymosan)-driven arthritic pain-like behaviour (herein referred to as pain) was completely dependent upon IL-23; optimal arthritic disease development required IL-23 (P < 0.05). Zymosan-induced inflammatory pain was also completely dependent on IL-23. In addition, we found that exogenous TNF-, GM-CSF-, and CCL17-driven arthritic pain, as well as inflammatory pain driven by each of these cytokines, were absent in Il23p19−/− mice; optimal disease in these mBSA-primed models was dependent on IL-23 (P < 0.05). Supporting this cytokine connection, it was found conversely that IL-23 (200 ng) can induce inflammatory pain at 4 h (P < 0.0001) with a requirement for each of the other cytokines as well as cyclooxygenase activity. These findings indicate a role for IL-23 in innate immune-mediated arthritic and inflammatory pain with potential links to TNF, GM-CSF, CCL17, and eicosanoid function.

|
Scooped by
Gilbert C FAURE
February 28, 2020 2:07 PM
|
JOURNAL ARTICLE Establishment of anti-C1q monoclonal antibodies to measure serum C1q levels discriminating disease severity subsets of rheumatoid arthritis within 5 years of onset Chikako Yukioka, Kosuke Ebina, Yasunori Shimaoka, Masao Yukioka, Hideki Yoshikawa, Ken Nakata, Takahiro Ochi Modern Rheumatology 2019 September 19, : 1-5 Objectives: To establish anti-C1q monoclonal antibodies which can measure serum C1q levels discriminating disease severity subsets of rheumatoid arthritis (RA) within 5 years of onset. Methods: In this multi-centre, longitudinal, observational study, 122 RA patients [102 females, baseline age 58.5 years, rheumatoid factor (RF) positivity 78.7%, serum C-reactive protein (CRP) 1.2 mg/dl, and concomitant methotrexate (MTX) 4.9 mg/week (29.5%)] within 5 years of onset (disease duration 21.0 months) were enrolled from 1985 to 2000. Patients were not treated by more than 8 mg/week of MTX or biologics which may strongly affect the course of joint destruction. Disease severity at 10-15 years of onset was classified according to the number of destructed joints of overall 68 joints on plain radiographs (36 patients were mild RA group involving only peripheral joints and 86 were severe RA group involving large axial joints). Baseline serum C1q levels were evaluated by ELISA with newly developed 4 monoclonal anti-C1q antibodies, and compared between two groups as well as conventional RA disease activity markers. Results: There were no significant differences between two groups in baseline conventional RA disease activity markers such as RF, erythrocyte sedimentation rate, CRP, and matrix metalloproteinase-3. However, compared to mild RA group, severe RA group showed higher baseline serum C1q levels (μg/ml) evaluated by anti-C1q monoclonal antibodies of no.33 (104.8 ± 22.3 vs. 118.3 ± 19.3; p = .0024), no. 40 (102.6 ± 21.9 vs 121.2 ± 22.3; p = .000069), no. 54 (102.1 ± 22.5 vs. 119.3 ± 26.9; p = .00052), and no. 76 (105.6 ± 21.8 vs. 122.6 ± 26.4; p = .00043). Receiver operating characteristic curve analysis revealed that in patients with serum C1q levels of ≥110.5 μg/ml (measured by antibody no. 40), 78.9% (75/95) belonged to severe RA group. Conclusion: Measuring serum C1q levels of RA within 5 years of onset by newly developed anti-C1q antibodies may be useful in predicting the prognosis of disease severity evaluated by the extent of joint destruction. Discussion You are not logged in. Sign Up or Log In to join the discussion. Related Papers Serum matrix metalloproteinase-3 as predictor of joint destruction in rheumatoid arthritis, treated with non-biological disease modifying anti-rheumatic drugs. Akira Mamehara, Takeshi Sugimoto, Daisuke Sugiyama, Sahoko Morinobu, Goh Tsuji, Seiji Kawano, Akio Morinobu, Shunichi Kumagai Kobe Journal of Medical Sciences 2010 September 30, 56 (3): E98-107 Effect of Sanhuangyilong decoction plus methotrexate on tumor necrosis factor alpha and interferon gamma in serum and synovial fluid in rheumatoid arthritis patients with symptom pattern of damp heat obstruction. Defang Liu, Jiao Yan, Mingdong Yun, Ming Yang, Yong Luo, Jun Zhang, Mingyang Guo, Mei Yang, Weili Yuan, Wei Zou, Hua Li, Yonghe Hu Journal of Traditional Chinese Medicine 2016, 36 (5): 625-33 The diagnostic utility of anti-cyclic citrullinated peptide antibodies, matrix metalloproteinase-3, rheumatoid factor, erythrocyte sedimentation rate, and C-reactive protein in patients with erosive and non-erosive rheumatoid arthritis. O Shovman, B Gilburd, G Zandman-Goddard, Y Sherer, H Orbach, R Gerli, Y Shoenfeld Clinical & Developmental Immunology 2005, 12 (3): 197-202 Unique correlation between mutated citrullinated vimentine IgG autoantibodies and markers of systemic inflammation in rheumatoid arthritis patients. Walid E Zahran, Magda I Mahmoud, Kamal A Shalaby, Manal H Abbas Indian Journal of Clinical Biochemistry: IJCB 2013, 28 (3): 272-6 Baseline serum level of matrix metalloproteinase-3 as a biomarker of progressive joint damage in rheumatoid arthritis patients. Sahar Mahfouz Abdel Galil, Abeer Mohamed El-Shafey, Hoda A Hagrass, Faten Fawzy, Ahmed El Sammak International Journal of Rheumatic Diseases 2016, 19 (4): 377-84 Rheumatoid Factor Is Associated With the Distribution of Hand Joint Destruction in Rheumatoid Arthritis. Chikashi Terao, Noriyuki Yamakawa, Koichiro Yano, Iris M Markusse, Katsunori Ikari, Shinji Yoshida, Moritoshi Furu, Motomu Hashimoto, Hiromu Ito, Takao Fujii, Koichiro Ohmura, Kosaku Murakami, Meiko Takahashi, Masahide Hamaguchi, Yasuharu Tabara, Atsuo Taniguchi, Shigeki Momohara, Soumya Raychaudhuri, Cornelia F Allaart, Hisashi Yamanaka, Tsuneyo Mimori, Fumihiko Matsuda Arthritis & Rheumatology 2015, 67 (12): 3113-23 The natural history and prognosis of rheumatoid arthritis: association of radiographic outcome with process variables, joint motion and immune proteins. Niels Graudal Scandinavian Journal of Rheumatology. Supplement 2004, 118: 1-38 Leucine-rich α2 -glycoprotein as a potential biomarker for joint inflammation during anti-interleukin-6 biologic therapy in rheumatoid arthritis. Minoru Fujimoto, Satoshi Serada, Katsuya Suzuki, Ayumi Nishikawa, Atsushi Ogata, Toshihiro Nanki, Kunihiro Hattori, Hitoshi Kohsaka, Nobuyuki Miyasaka, Tsutomu Takeuchi, Tetsuji Naka Arthritis & Rheumatology 2015, 67 (8): 2056-60 Matrix metalloproteinase-3 levels in relation to disease activity and radiological progression in rheumatoid arthritis. Turkan Tuncer, Arzu Kaya, Arif Gulkesen, Gul Ayden Kal, Dilara Kaman, Gurkan Akgol Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University 2019 February 8 Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review. Sushil Kaur, Sarahlouise White, Mark Bartold JBI Library of Systematic Reviews 2012, 10 (42 Suppl): 1-12

|
Scooped by
Gilbert C FAURE
February 20, 2020 6:44 AM
|
After decades of sometimes fierce debate about the advantages and disadvantages of glucocorticoids, an age of convergence has been reached. Current recommendations for the management of diseases such as rheumatoid arthritis (RA), polymyalgia rheumatica and large vessel vasculitis reflect the current consensus that as much glucocorticoid as necessary, but as little as possible, should be used. Over the past few years, a range of glucocorticoid-sparing strategies have been developed, as have tools to improve the management of this therapy. A comprehensive view of glucocorticoid-induced osteoporosis has also emerged that recognizes that bone fragility is not solely determined by the dose and duration of glucocorticoid treatment. Nevertheless, open questions remain around whether long-term use of very low doses of glucocorticoids is a realistic option for patients with RA and whether the search for innovative glucocorticoids or glucocorticoid receptor ligands with improved benefit-to-risk ratios will ultimately be successful. Glucocorticoids have been used to treat patients with rheumatic diseases for more than 70 years, but have been controversial owing to their safety record. Are we now entering an age when opinions about their use in rheumatology clinics are converging?
The Wnt signaling pathway plays a key role in several biological processes, such as cellular proliferation and tissue regeneration, and its dysregulation is involved in the pathogenesis of many autoimmune diseases.
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
October 10, 2019 12:46 PM
|
MRI of the sacroiliac joints is increasingly acknowledged as being indispensable in the early diagnosis of axial spondyloarthritis (axSpA) and as having a prominent role in the prognosis and classification of axSpA. Technological advances include improvements in the resolution of structural lesions and in methodologies for the quantification of lesions. Limited access and expertise in interpretation of MRI have led to a resurgence of interest in CT, especially the development of low radiation protocols for assessing the sacroiliac joints. Trials of TNF inhibitors in patients with non-radiographic axSpA have led to greater understanding of the role of MRI in selecting which patients might respond well to this therapy. The role of MRI features as target end points in treat-to-target strategies remains unclear because the effect of such targeting on structural damage parameters has only recently been explored. The relative importance of active and structural lesions for prognostic risk assessment and selection of appropriate treatment is also an area of current research. Given the increased capacity to visualize a broad array of lesions in both the sacroiliac joints and the spine using MRI and CT, these modalities will probably be increasingly employed for assessment of the disease-modifying activity of new therapies. Different imaging modalities, such as radiography, MRI and CT, have different advantages and can help the clinician with different aspects in assessing axial spondyloarthritis (axSpA). This Review covers imaging aspects relating to the diagnosis, classification and management of axSpA.
|

|
Scooped by
Gilbert C FAURE
July 23, 2024 3:30 AM
|
Background The increased availability of myositis autoantibodies represents new possibilities and challenges in clinical practice (Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76:1955–64. https://doi.org/10.1136/annrheumdis-2017-211468 .). The aim of this study was to perform a retrospective data analysis of patient cases with positive myositis autoantibodies to analyse their significance in routine rheumatology practice. Methods A monocentric analysis of all the orders used to determine myositis autoantibodies from July 2019 to May 2022 in the Department of Rheumatology, Krankenhaus Porz am Rhein, Cologne, Germany, was carried out. Results In the defined time interval, a total of 71,597 laboratory values for the antibodies mentioned above were obtained. A total of 238 different positive autoantibodies were detected in 209 patients. Idiopathic inflammatory myopathy was diagnosed in 37 patients (18%), and inflammatory rheumatic diseases other than idiopathic inflammatory myopathy were diagnosed in 90 patients (43%). No inflammatory rheumatic disease was diagnosed in 82 patients (39%). General clusters of clinical manifestations were observed. Conclusions In our cohort, we were able to show that a relevant proportion of patients with positive myositis antibodies did not have idiopathic inflammatory myopathies or inflammatory rheumatic diseases. This finding indicates the importance of myositis autoantibodies in this group of patients. However, further studies on the course of symptoms and examination results in patients without inflammatory rheumatic diseases and with positive myositis antibodies are necessary.

|
Scooped by
Gilbert C FAURE
June 17, 2023 7:02 AM
|
Audio Rheum Editor’s Picks Dr. Earl D. Silverman, MD, shares his monthly Editor’s Picks and their relevance in current clinical practice: June 2023 Featured articles: Kiltz, et al: Clinimetric Validation of the Assessment of Spondyloarthritis International Society Health Index in Patients With Radiographic Axial Spondyloarthritis in Ixekizumab Trials Alduraibi, et al: Clustering Patients With Gout Based on Comorbidities and Biomarkers: A Cross-Sectional Study Primeau, et al: Responders to Medial Opening Wedge High Tibial Osteotomy for Knee Osteoarthritis Kumthekar, et al: Physical Activity Habits Among Older Adults Living With Rheumatic Disease Wohlfahrt, et al: Pain Mechanisms Associated With Disease Activity in Patients With Rheumatoid Arthritis Treated With Disease-Modifying Antirheumatic Drugs: A Regression Tree Analysis May 2023 Featured articles: Cook, et al: Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines Against COVID-19 Infection Among Patients With Systemic Autoimmune Rheumatic Diseases on Immunomodulatory Medications Chen, et al: Validation of the Antineutrophil Cytoplasmic Antibody Renal Risk Score and Modification of the Score in a Chinese Cohort With a Majority of Myeloperoxidase-Positive Patients Gorzewski, et al: Predicting Disease Activity in Rheumatoid Arthritis With the Fibromyalgia Survey Questionnaire: Does the Severity of Fibromyalgia Symptoms Matter? Oguro, et al: Effect of Communicative and Critical Health Literacy on Trust in Physicians Among Patients With Systemic Lupus Erythematosus (SLE): The TRUMP2-SLE Project Barber, et al: Investigating Associations Between Access to Rheumatology Care, Treatment, Continuous Care, and Healthcare Utilization and Costs Among Older Individuals With Rheumatoid Arthritis April 2023 Featured articles: Coates, et al: Sex-Specific Differences in Patients With Psoriatic Arthritis: A Systematic Review Johnson, et al: Evaluating the Threshold Score for Classification of Systemic Lupus Erythematosus Using the EULAR/ACR Criteria Gong, et al: The Association Between Quadriceps Strength and Synovitis in Knee Osteoarthritis: An Exploratory Study From the Osteoarthritis Initiative Jatuworapruk, et al: Prevalence, Risk Factors, and Outcomes of Gout Flare in Patients Hospitalized for PCR-Confirmed COVID-19: A Multicenter Retrospective Cohort Study Bosch, et al: Etanercept Withdrawal and Retreatment in Nonradiographic Axial Spondyloarthritis: Results of RE-EMBARK, an Open-Label Phase IV Trial March 2023 Featured articles: Takanashi, et al: Effects of Aging on Rheumatoid Factor and Anticyclic Citrullinated Peptide Antibody Positivity in Patients With Rheumatoid Arthritis Kiltz, et al: Clinically Relevant Deficits in Performance Tests in Patients With Axial Spondyloarthritis Schletzbaum, et al: Age-Stratified 30-day Rehospitalization and Mortality and Predictors of Rehospitalization Among Patients With Systemic Lupus Erythematosus: A Medicare Cohort Study Berard, et al: Canadian Rheumatology Association Recommendations for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis Schultz, et al: B Cell Reconstitution is Associated With COVID-19 Booster Vaccine Responsiveness in Patients Previously Seronegative Treated With Rituximab February 2023 Featured articles: Macfarlane, et al: Inflammatory Bowel Disease Risk in Patients With Axial Spondyloarthritis Treated With Biologic Agents Determined Using the BSRBR-AS and a MetaAnalysis Gossec, et al: Women With Psoriatic Arthritis Experience Higher Disease Burden Than Men: Findings From a Real-World Survey in the United States and Europe Davis, et al: The Effect of Psychiatric Comorbidity on Healthcare Utilization for Youth With Newly Diagnosed Systemic Lupus Erythematosus Pyo, et al: The Reclassification of Patients With Previously Diagnosed Eosinophilic Granulomatosis With Polyangiitis Based on the 2022 ACR/EULAR Criteria for Antineutrophil Cytoplasmic Antibody–Associated Vasculitis Weng, et al: Adult-Onset Still Disease After ChAdOx1 nCOV-19 Vaccination Mitchell, et al: How to Provide Sexual and Reproductive Health Care to Patients: Focus Groups With Rheumatologists and Rheumatology Advanced Practice Providers January 2023 Featured articles: Kodishala, et al: Risk Factors for Dementia in Patients With Incident Rheumatoid Arthritis: A Population-Based Cohort Study Beauvais, et al: Development and Validation of a Self-Administered Questionnaire Measuring Essential Knowledge in Patients With Axial Spondyloarthritis Orbai, et al: Impact of Physician-Defined Flares on Quality of Life and Work Impairment: An International Survey of 2238 Patients With Psoriatic Arthritis Smitherman, et al: Patient-Reported Outcomes Among Transition-Age Young Adults With Juvenile Idiopathic Arthritis in the Childhood Arthritis and Rheumatology Research Alliance Registry Stull, et al: Cutaneous Involvement in Systemic Lupus Erythematosus: A Review for the Rheumatologist Masi, et al: Reflections for the 50th Anniversary of The Journal of Rheumatology: The Past, Present, and Future of Rheumatology Santos, et al: A Rare Case of Subcutaneous Sarcoidosis in Patient With Psoriatic Arthritis Garg, et al: Timing and Predictors of Incident Cardiovascular Disease in Systemic Lupus Erythematosus: Risk Occurs Early and Highlights Racial Disparities December 2022 Featured articles: Koo, et al: Relationship Between Inflammation and Radiographic Progression in Patients With Ankylosing Spondylitis Attaining a BASDAI of Less Than 4 During Tumor Necrosis Factor Inhibitor Treatment Sun, et al: Development and Initial Validation of a Systemic Lupus Erythematosus–Specific Measure of the Extent of and Reasons for Medication Nonadherence Patterson, et al: Physical Activity Associates With Lower Systemic Inflammatory Gene Expression in Rheumatoid Arthritis Coleman, et al: Long-Term Follow-up of a Randomized Controlled Trial of Allopurinol Dose Escalation to Achieve Target Serum Urate in People With Gout Smith: Rusty and Wooden Tanomogi, et al: Extravascular Necrotizing Granuloma: A Diagnostic Clue for Eosinophilic Granulomatosis With Polyangiitis Isnardi, et al: Immune Response to SARS-CoV-2 Third Vaccine in Patients With Rheumatoid Arthritis Who Had No Seroconversion After Primary 2-Dose Regimen With Inactivated or Vector-Based Vaccines November 2022 Featured articles: Nelson, et al: Narrative Review of Machine Learning in Rheumatic and Musculoskeletal Diseases for Clinicians and Researchers: Biases, Goals, and Future Directions Hermans, et al: Are All Routine Spondyloarthritis Outpatient Visits Considered Useful by Rheumatologists? An Exploratory Clinical Practice Study Kallas, et al: Trajectory of Damage Accrual in Systemic Lupus Erythematosus Based on Ethnicity and Socioeconomic Factors Nozawa, et al: Early Abnormal Nailfold Capillary Changes Are Predictive of Calcinosis Development in Juvenile Dermatomyositis Chevet, et al: COVID-19 Vaccine Uptake Among Patients With Systemic Lupus Erythematosus in the American Midwest: The Lupus Midwest Network (LUMEN) Remize, et al: Melorheostosis or “Dripping Candle Wax” Bone Disease Bermas: The Unintended Consequence of the Overturn of Roe v Wade: Restrictions on Methotrexate Use October 2022 Featured articles: Hazlewood, et al: Canadian Rheumatology Association Living Guidelines for the Pharmacological Management of Rheumatoid Arthritis With Disease-Modifying Antirheumatic Drugs Schneeberger, et al: Simplified Ankylosing Spondylitis Disease Activity Score (SASDAS) Versus ASDAS: A Post Hoc Analysis of a Randomized Controlled Trial Kasiem, et al: A Practical Guide for Assessment of Skin Burden in Patients With Psoriatic Arthritis Tollisen, et al: Personally Generated Quality of Life Outcomes in Adults With Juvenile Idiopathic Arthritis Glintborg, et al: Long-term Behavioral Changes During the COVID-19 Pandemic and Impact of Vaccination in Patients With Inflammatory Rheumatic Diseases September 2022 Featured articles: Cagnotto, et al: Male Sex Predicts a Favorable Outcome in Early ACPA-Negative Rheumatoid Arthritis: Data From an Observational Study Hazlewood, et al: Frequency of Symptomatic Adverse Events in Rheumatoid Arthritis: An Exploratory Online Survey John M. Davis III: The Patient Experience of Drug Side Effects in Rheumatoid Arthritis: Intriguing Data From an Exploratory Online Survey Venkatachalam, et al: Taking the Long View: Patients Perceive Benefits and Risks of Treatment as Multidimensional Wallace, et al: The Association of Illness-related Uncertainty With Mental Health in Systemic Autoimmune Rheumatic Diseases August 2022 Featured articles: Verweij, et al: Whole-Body Macrophage Positron Emission Tomography Imaging for Disease Activity Assessment in Early Rheumatoid Arthritis Nguyen, et al: Secukinumab in United States Biologic-Naïve Patients With Psoriatic Arthritis: Results From the Randomized, Placebo-Controlled CHOICE Study Le Ralle, et al: Patient Acceptable Symptom State for Burden From Appearance Changes in People With Systemic Sclerosis: A Cross-sectional Survey Baggett, et al: Incidence Rates of Psoriasis in Children With Inflammatory Bowel Disease and Juvenile Arthritis Treated With Tumor Necrosis Factor Inhibitors and Disease-Modifying Antirheumatic Drugs Carluzzo, et al: Patient Empowerment Among Adults With Arthritis: The Case for Emotional Support July 2022 Featured articles: Kelty, et al: Mortality Rates in Patients With Ankylosing Spondylitis With and Without Extraarticular Manifestations and Comorbidities: A Retrospective Cohort Study Mease, et al: Baseline Disease Activity Predicts Achievement of cDAPSA Treatment Targets With Apremilast: Phase III Results in DMARD-naïve Patients With Psoriatic Arthritis Kuwana, et al: Tacrolimus in Patients With Interstitial Pneumonia Associated With Polymyositis or Dermatomyositis: Interim Report of Postmarketing Surveillance in Japan Kiadaliri, et al: Gout and Hospital Admission for Ambulatory Care–Sensitive Conditions: Risks and Trajectories McDermott, et al: Demographic, Lifestyle, and Serologic Risk Factors for Rheumatoid Arthritis (RA)–associated Bronchiectasis: Role of RA-related Autoantibodies June 2022 Featured articles: Darabian, et al: Using FibroScan to Assess for the Development of Liver Fibrosis in Patients With Arthritis on Methotrexate: A Single-center Experience Maguire, et al: Central Obesity in Axial Spondyloarthritis: The Missing Link to Understanding Worse Outcomes in Women? Miloslavsky, et al: The Challenge of Addressing the Rheumatology Workforce Shortage Maheswaranathan, et al: Association of Health Literacy and Numeracy With Lupus Knowledge and the Creation of the Lupus Knowledge Assessment Test Emad, et al: Why Do Patients With Gout Not Take Allopurinol? May 2022 Featured articles: Movahedi, et al: Physician- and Patient-reported Effectiveness Are Similar for Tofacitinib and TNFi in Rheumatoid Arthritis: Data From a Rheumatoid Arthritis Registry El Tal, et al: Consensus Approach to a Treat-to-target Strategy in Juvenile Idiopathic Arthritis Care: Report From the 2020 PR-COIN Consensus Conference Thompson, et al: Modifiable Factors and Incident Gout Across Ethnicity Within a Large Multiethnic Cohort of Older Adults Widdifield, et al: COVID-19 Vaccination Uptake Among Individuals With Immune-mediated Inflammatory Diseases in Ontario, Canada, Between December 2020 and October 2021: A Population-based Analysis Van Praet, et al: Acute Perimyocarditis in a Case of Multisystem Inflammatory Syndrome in Adults Chang, et al: Systemic Lupus Erythematosus Increases the Risk of Gestational Diabetes: Truth or Illusion? McCormick and Choi: Racial Disparities in the Modern Gout Epidemic April 2022 Featured articles: Exarchou, et al: Lifestyle Factors and Disease Activity Over Time in Early Axial Spondyloarthritis: The SPondyloArthritis Caught Early (SPACE) Cohort - https://doi.org/10.3899/jrheum.210046 van Vollenhoven, et al: Efficacy and Safety of Ustekinumab in Patients With Active Systemic Lupus Erythematosus: Results of a Phase II Open-label Extension Study - https://doi.org/10.3899/jrheum.210805 Giancane, et al: Anakinra in Patients With Systemic Juvenile Idiopathic Arthritis: Long-term Safety From the Pharmachild Registry - https://doi.org/10.3899/jrheum.210563 Barber, et al: Best Practices for Virtual Care: A Consensus Statement From the Canadian Rheumatology Association - https://doi.org/10.3899/jrheum.211017 Zickuhr and Mandell: Rheumatology Education Needs a Splash of Color - https://doi.org/10.3899/jrheum.211233 Li, et al: Brain Abscess Due to Nocardia in a Patient With Systemic Lupus Erythematosus- https://doi.org/10.3899/jrheum.210971 March 2022 Featured articles: te Kampe, et al: Outcomes of Care Among Patients With Gout in Europe: A Cross-sectional Survey - https://doi.org/10.3899/jrheum.210009 Stransky, et al: Exploring Family Planning, Parenting, and Sexual and Reproductive Health Care Experiences of Men With Rheumatic Diseases - https://doi.org/10.3899/jrheum.210785 Beckers, et al: Performance of 3 Composite Measures for Disease Activity in Peripheral Spondyloarthritis - https://doi.org/10.3899/jrheum.210075 Sun, et al: Long-term Risk of Heart Failure and Other Adverse Cardiovascular Outcomes in Granulomatosis With Polyangiitis: A Nationwide Cohort Study - https://doi.org/10.3899/jrheum.210677 Curtis, et al: Characteristics, Comorbidities, and Outcomes of SARS-CoV-2 Infection in Patients With Autoimmune Conditions Treated With Systemic Therapies: A Population-based Study - https://doi.org/10.3899/jrheum.210888 Gunasuntharam: Why Should It Be Different From the Other Side? A Parent and Pediatrician’s Perspective of a Child With Kawasaki Disease - https://doi.org/10.3899/jrheum.211159 Lazarou, et al: Adult-onset Acute Calcific Discitis - https://doi.org/10.3899/jrheum.210838 Khanna: Remission in Gout: Concepts From a Patient Perspective - https://doi.org/10.3899/jrheum.211285 Coates and Tillett: How Should We Measure Peripheral Spondyloarthritis? - https://doi.org/10.3899/jrheum.211043 February 2022 Featured articles: Rosenbaum, et al: The Interplay Between COVID-19 and Spondyloarthritis or Its Treatment - https://doi.org/10.3899/jrheum.210742 van der Heijde, et al: Radiographic Progression of Structural Joint Damage Over 5 Years of Baricitinib Treatment in Patients With Rheumatoid Arthritis: Results From RA-BEYOND - https://doi.org/10.3899/jrheum.210346 van der Meer, et al: Extraskeletal Manifestations in Axial Spondyloarthritis Are Associated With Worse Clinical Outcomes Despite the Use of Tumor Necrosis Factor Inhibitor Therapy - https://doi.org/10.3899/jrheum.210308 Haddad, et al: The Association of Psoriatic Arthritis With All-cause Mortality and Leading Causes of Death in Psoriatic Arthritis - https://doi.org/10.3899/jrheum.210159 Yazdanyar, et al: Risk of 30-day Readmission After Knee or Hip Replacement in Rheumatoid Arthritis and Osteoarthritis by Non-Medicare and Medicare Payer Status - https://doi.org/10.3899/jrheum.201370 Perez Acosta et al: Cystic Ganglionosis in a 3-year-old Mimicking Juvenile Idiopathic Arthritis - https://doi.org/10.3899/jrheum.210558 Leung: Is Psoriatic Arthritis Associated With Higher Risk of Mortality? - https://doi.org/10.3899/jrheum.210963 January 2022 Featured articles: Bermas, et al: COVID-19 in Pregnant Women With Rheumatic Disease: Data From the COVID-19 Global Rheumatology Alliance - https://doi.org/10.3899/jrheum.210480 Hazlewood, et al: Heterogeneity in Patient Characteristics and Differences in Treatment Across 4 Canadian Rheumatoid Arthritis Cohorts - https://doi.org/10.3899/jrheum.201688 Panopoulos, et al: Anti-interleukin 6 Therapy Effect for Refractory Joint and Skin Involvement in Systemic Sclerosis: A Real-world, Single-center Experience - https://doi.org/10.3899/jrheum.210273 Lieber, et al: Evaluation of a Patient-reported Frailty Tool in Women With Systemic Lupus Erythematosus - https://doi.org/10.3899/jrheum.201466 Philpott, et al: Synovitis Is Associated With Constant Pain in Knee Osteoarthritis: A Cross-sectional Study of OMERACT Knee Ultrasound Scores - https://doi.org/10.3899/jrheum.210285 Fernandes, et al: Tuberculosis Presenting as an Inflammatory Pseudotumor of the Sciatic Nerve in a Rheumatoid Arthritis Patient Taking Etanercept - https://doi.org/10.3899/jrheum.210540 Sammaritano: The Effect of COVID-19 Illness on Pregnant Patients With Rheumatic Disease: Early Reassuring Data - https://doi.org/10.3899/jrheum.211050 December 2021 Featured articles: Kremer, et al: The Clinical Disease Activity Index and the Routine Assessment of Patient Index Data 3 for Achievement of Treatment Strategies - https://doi.org/10.3899/jrheum.200692 Müskens, et al: Does Etanercept Biosimilar Prescription in a Rheumatology Center Bend the Medication Cost Curve? - https://doi.org/10.3899/jrheum.200565 Gladman, et al: Oligoarticular vs Polyarticular Psoriatic Arthritis: A Longitudinal Study Showing Similar Characteristics - https://doi.org/10.3899/jrheum.210434 Showalter, et al: Esophageal Dilation and Other Clinical Factors Associated With Pulmonary Function Decline in Patients With Systemic Sclerosis - https://doi.org/10.3899/jrheum.210533 Holyer, et al: What Represents Treatment Efficacy in Long-term Studies of Gout Flare Prevention? An Interview Study of People With Gout - https://doi.org/10.3899/jrheum.210476 Tam et al: My Hips Hurt: An Unusual Presentation of Bilateral Groin Pain in an Adolescent Boy - https://doi.org/10.3899/jrheum.210362 Ferreira, et al: Definition of Treatment Targets in Rheumatoid Arthritis: Is It Time for Reappraisal? - https://doi.org/10.3899/jrheum.210050 Jacobs, et al: Unravelling the Cost of Biological Strategies in Rheumatoid Arthritis: A Kaleidoscope of Methodologies, Interpretations, and Interests - https://doi.org/10.3899/jrheum.201510 November 2021 Featured articles: Colantonio, et al: Higher Serum Urate Levels Are Associated With an Increased Risk for Sudden Cardiac Death - https://doi.org/10.3899/jrheum.210139 Ochi, et al: Similarity of Response to Biologics Between Elderly-onset Rheumatoid Arthritis (EORA) and Non-EORA Elderly Patients: From the FIRST Registry - https://doi.org/10.3899/jrheum.201135 Tanaka, et al: Effects of Denosumab in Japanese Patients With Rheumatoid Arthritis Treated With Conventional Antirheumatic Drugs: 36-month Extension of a Phase III Study - https://doi.org/10.3899/jrheum.201376 Ye, et al: Measuring Physical Function in Psoriatic Arthritis: Comparing the Multidimensional Health Assessment Questionnaire to the Health Assessment Questionnaire–Disability Index - https://doi.org/10.3899/jrheum.200927 Concha, et al: Changes in Treatments and Outcomes After Implementation of a National Universal Access Program for Juvenile Idiopathic Arthritis - https://doi.org/10.3899/jrheum.210011 Meara, et al: A Case of Chilblains-like Lesions Post SARS-CoV-2 Vaccine? - https://doi.org/10.3899/jrheum.210226 Liu: Serum Uric Acid: A Murderer or Bystander for Cardiac-related Mortality? - https://doi.org/10.3899/jrheum.210695 Berard and Batthish: Addressing Healthcare Quality in Juvenile Idiopathic Arthritis With a Universal Access Program - https://doi.org/10.3899/jrheum.210658 October 2021 Featured articles: Safy-Khan, et al: Current Smoking Negatively Affects the Response to Methotrexate in Rheumatoid Arthritis in a Dose-responsive Way, Independently of Concomitant Prednisone Use - https://doi.org/10.3899/jrheum.200213 Mease, et al: Comparison of Men and Women With Axial Spondyloarthritis in the US-based Corrona Psoriatic Arthritis/Spondyloarthritis Registry - https://doi.org/10.3899/jrheum.201549 Feher, et al: Impaired Myocardial Flow Reserve on 82Rubidium Positron Emission Tomography/Computed Tomography in Patients With Systemic Sclerosis - https://doi.org/10.3899/jrheum.210040 Mohajer, et al: Metabolic Syndrome and Osteoarthritis Distribution in the Hand Joints: A Propensity Score Matching Analysis From the Osteoarthritis Initiative - https://doi.org/10.3899/jrheum.210189 Fernández-Ávila, et al: Impact of COVID-19 Pandemic on Rheumatology Practice in Latin America - https://doi.org/10.3899/jrheum.201623https://doi.org/10.3899/jrheum.201623 Gilvaz et al: A Case of Disseminated Cutaneous Mycobacterium chelonae Infection During Treatment With Tofacitinib - https://doi.org/10.3899/jrheum.200730 Jansen, et al: Smoking and Methotrexate Inefficacy in Rheumatoid Arthritis: What About Underlying Molecular Mechanisms? - https://doi.org/10.3899/jrheum.210217 Leung: Gender Differences in Disease Activity and Impact in Axial Spondyloarthritis - https://doi.org/10.3899/jrheum.210564 September 2021 Featured articles: Myasoedova, et al: Improved Incidence of Cardiovascular Disease in Patients With Incident Rheumatoid Arthritis in the 2000s: A Population-based Cohort Study - https://doi.org/10.3899/jrheum.200842 Karmacharya, et al: Diagnostic Delay in Psoriatic Arthritis: A Population-based Study - https://doi.org/10.3899/jrheum.201199 Clément, et al: Real-world Risk of Relapse of Giant Cell Arteritis Treated With Tocilizumab: A Retrospective Analysis of 43 Patients - https://doi.org/10.3899/jrheum.200595 Master, et al: Joint Association of Moderate-to-vigorous Intensity Physical Activity and Sedentary Behavior With Incident Functional Limitation: Data From the Osteoarthritis Initiative - https://doi.org/10.3899/jrheum.201250 Sheth et al: Improving Pneumococcal Vaccination Rates in Rheumatology Patients by Using Best Practice Alerts in the Electronic Health Records - https://doi.org/10.3899/jrheum.200806 Bathon: Is the Gap in Incidence of Cardiovascular Events in Rheumatoid Arthritis Really Closing? - https://doi.org/10.3899/jrheum.210366 Villiger: Giant Cell Arteritis: Real-life Experience - https://doi.org/10.3899/jrheum.210334 Gazitt, et al: Spinal Stenosis Caused by Calcinosis in a Patient With Systemic Sclerosis - https://doi.org/10.3899/jrheum.201389 De Boer and Goekoop: Posttraumatic Chylous Knee Effusion - https://doi.org/10.3899/jrheum.191050 August 2021 Featured articles: Vu, et al: Impact of Comorbid Conditions on Healthcare Expenditure and Work-related Outcomes in Patients With Rheumatoid Arthritis - https://doi.org/10.3899/jrheum.200231 Taylor, et al: A Phase III Randomized Study of Apremilast, an Oral Phosphodiesterase 4 Inhibitor, for Active Ankylosing Spondylitis - https://doi.org/10.3899/jrheum.201088 Falasinnu, et al: The Problem of Pain in Systemic Lupus Erythematosus: An Explication of the Role of Biopsychosocial Mechanisms - https://doi.org/10.3899/jrheum.200595 Hazlewood, et al: Canadian Rheumatology Association Recommendation for the Use of COVID-19 Vaccination for Patients With Autoimmune Rheumatic Diseases - https://doi.org/10.3899/jrheum.210288 Aljaberi et al: Maintaining Hepatitis B Protection in Immunocompromised Pediatric Rheumatology and Inflammatory Bowel Disease Patients - https://doi.org/10.3899/jrheum.200283 Bechman, et al: The COVID-19 Vaccine Landscape: What a Rheumatologist Needs to Know - https://doi.org/10.3899/jrheum.210106 Rahman: Why Do Patients With Systemic Lupus Erythematosus Suffer Pain? - https://doi.org/10.3899/jrheum.210057 Awqati, et al: Ulcerative Paraneoplastic Dermatomyositis in the Setting of Positive Transcriptional Intermediary Factor 1-γ Antibody - https://doi.org/10.3899/jrheum.200399 Shinoda, et al: Widespread Mechanic’s Hands in Antisynthetase Syndrome With Anti-OJ Antibody - https://doi.org/10.3899/jrheum.201043 July 2021 Featured articles: Pathi, et al: The Rheumatoid Arthritis Gene Expression Signature Among Women Who Improve or Worsen During Pregnancy: A Pilot Study - https://doi.org/10.3899/jrheum.201128 Stovall, et al: Relation of NSAIDs, DMARDs, and TNF Inhibitors for Ankylosing Spondylitis and Psoriatic Arthritis to Risk of Total Hip and Knee Arthroplasty - https://doi.org/10.3899/jrheum.200453 Mehta, et al: Giant Cell Arteritis and COVID-19: Similarities and Discriminators. A Systematic Literature Review - https://doi.org/10.3899/jrheum.200766 Widdifield, et al: Feminization of the Rheumatology Workforce: A Longitudinal Evaluation of Patient Volumes, Practice Sizes, and Physician Remuneration - https://doi.org/10.3899/jrheum.201166 Bachiller-Corral et al: Risk of Severe COVID-19 Infection in Patients With Inflammatory Rheumatic Diseases - https://doi.org/10.3899/jrheum.200755 Ornetti, et al: Perforating Rheumatoid Nodule Mimicking Malignant Soft-tissue Mass of the Forearm - https://doi.org/10.3899/jrheum.201290 Guillaune-Czitrom, et al: A Recurrent Central Band Keratopathy in a Child - https://doi.org/10.3899/jrheum.200462 June 2021 Featured articles: Xie, et al: Benefits of Methotrexate Use on Cardiovascular Disease Risk Among Rheumatoid Arthritis Patients Initiating Biologic Disease-modifying Antirheumatic Drugs - https://doi.org/10.3899/jrheum.191326 Jørgensen, et al: Relation Between Fatigue and ACR Response in Patients With Psoriatic Arthritis Treated With Tumor Necrosis Factor Inhibitor Therapy: A Population-based Cohort Study - https://doi.org/10.3899/jrheum.191107 Dominguez, et al: Relationship Between Genetic Risk and Age of Diagnosis in Systemic Lupus Erythematosus - https://doi.org/10.3899/jrheum.200002 Coffey, et al: Hospitalization Rates Are Highest in the First 5 Years of Systemic Sclerosis: Results From a Population-based Cohort (1980–2016) - https://doi.org/10.3899/jrheum.200737 Singh and Cleveland: Hospitalized Infections in People With Osteoarthritis: A National US Study - https://doi.org/10.3899/jrheum.191383 May 2021 Featured articles: Ozen, et al: The Risk of Cardiovascular Events Associated With Disease-modifying Antirheumatic Drugs in Rheumatoid Arthritis - doi.org/10.3899/jrheum.200265 Ogdie, et al: Descriptive Comparisons of the Effect of Apremilast and Methotrexate Monotherapy in Oligoarticular Psoriatic Arthritis: The Corrona Psoriatic Arthritis/Spondyloarthritis Registry Results - doi.org/10.3899/jrheum.191209 Kim, et al: Lupus Low Disease Activity State Achievement Is Important for Reducing Adverse Outcomes in Pregnant Patients With Systemic Lupus Erythematosus - doi.org/10.3899/jrheum.200802 Mossel, et al: Clinical Phenotyping of Primary Sjögren Syndrome Patients Using Salivary Gland Ultrasonography: Data From the RESULT Cohort - doi.org/10.3899/jrheum.200482 Panwar et al: Whole-body MRI Quantification for Assessment of Bone Lesions in Chronic Nonbacterial Osteomyelitis Patients Treated With Pamidronate: A Prevalence, Reproducibility, and Responsiveness Study - doi.org/10.3899/jrheum.200329 April 2021 Featured articles: Barber, et al: Evaluating Quality of Care for Rheumatoid Arthritis for the Population of Alberta Using System-level Performance Measures - doi.org/10.3899/jrheum.200420 Liu, et al: Physical Activity and Attitudes Toward Exercise in People With Axial and Peripheral Spondyloarthritis - doi.org/10.3899/jrheum.200354 Harkey, et al: A Decline in Walking Speed Is Associated With Incident Knee Replacement in Adults With and at Risk for Knee Osteoarthritis - doi.org/10.3899/jrheum.200176 Mendel, et al: CanVasc Consensus Recommendations for the Management of Antineutrophil Cytoplasm Antibody-associated Vasculitis: 2020 Update - doi.org/10.3899/jrheum.200721 Gkrouzman, et al: Antiphospholipid Antibody Profile Stability Over Time: Prospective Results From the APS ACTION Clinical Database and Repository - doi.org/10.3899/jrheum.200513 March 2021 Featured articles: Almaghlouth, et al: Propensity Score Methods in Rare Disease: A Demonstration Using Observational Data in Systemic Lupus Erythematosus - doi.org/10.3899/jrheum.200254 Faye, et al: Risk of Adverse Outcomes in Hospitalized Patients With Autoimmune Disease and COVID-19: A Matched Cohort Study From New York City - doi.org/10.3899/jrheum.200989 Grosse, et al: Evaluation of Bone Erosions in Rheumatoid Arthritis: The Ultrasound Score for Erosions Versus the Modified Sharp/van der Heijde Score for Erosions - doi.org/10.3899/jrheum.200286 Liew, et al: Cardiovascular Risk Scores in Axial Spondyloarthritis Versus the General Population: A Cross-sectional Study - doi.org/10.3899/jrheum.200188 van Leeuwen, et al: Association Between Centromere- and Topoisomerase-specific Immune Responses and the Degree of Microangiopathy in Systemic Sclerosis - doi.org/10.3899/jrheum.191331 February 2021 Featured articles: Kiltz, et al: Ixekizumab Improves Functioning and Health in the Treatment of Radiographic Axial Spondyloarthritis: Week 52 Results from 2 Pivotal Studies - doi.org/10.3899/jrheum.200093 Sarabia, et al: The Pattern of Musculoskeletal Complaints in Patients With Suspected Psoriatic Arthritis and Their Correlation With Physical Examination and Ultrasound - doi.org/10.3899/jrheum.190857 te Kampe, et al: Sex Differences in the Clinical Profile Among Patients With Gout: Cross-sectional Analyses of an Observational Study - doi.org/10.3899/jrheum.200113 Walscheid, et al: Enthesitis-related Arthritis: Prevalence and Complications of Associated Uveitis in Children and Adolescents From a Population-based Nationwide Study in Germany - doi.org/10.3899/jrheum.191085 Master, et al: Does the 1-year Decline in Walking Speed Predict Mortality Risk Beyond Current Walking Speed in Adults With Knee Osteoarthritis? - doi.org/10.3899/jrheum.200259 January 2021 Featured articles: Fisher, et al: Tofacitinib Persistence in Patients with Rheumatoid Arthritis: A Retrospective Cohort Study - doi.org/10.3899/jrheum.191252 Thomas, et al: Tumor Necrosis Factor Inhibitor Monotherapy Versus Combination Therapy for the Treatment of Psoriatic Arthritis: Combined Analysis of European Biologics Databases - doi.org/10.3899/jrheum.190815 Blaja, et al: The Challenge of Very Early Systemic Sclerosis: A Combination of Mild and Early Disease? - doi.org/10.3899/jrheum.190976 Zhai, et al: Phenylalanine Is a Novel Marker for Radiographic Knee Osteoarthritis Progression: The MOST Study - doi.org/10.3899/jrheum.200054 Koppikar, et al: Improving Hydroxychloroquine Dosing and Toxicity Screening at a Tertiary Care Ambulatory Center: A Quality Improvement Initiative - doi.org/10.3899/jrheum.191265 December 2020 Featured articles: Foers, et al: Circulating Small Noncoding RNA Biomarkers of Response to Triple Disease-modifying Antirheumatic Drug Therapy in White Women With Early Rheumatoid Arthritis - doi.org/10.3899/jrheum.191012 Wade, et al: Serum MicroRNA Signature as a Diagnostic and Therapeutic Marker in Patients with Psoriatic Arthritis - doi.org/10.3899/jrheum.190602 Schwartz, et al: Utility of the Brief Illness Perception Questionnaire to Monitor Patient Beliefs in Systemic Vasculitis - doi.org/10.3899/jrheum.190828 Correll, et al: Identifying Research Priorities among Patients and Families of Children with Rheumatic Diseases Living in the United States - doi.org/10.3899/jrheum.190934 Bitar, et al: Five-year Evolution Patterns of Physical Activity and Sedentary Behavior in Patients with Lower-limb Osteoarthritis and Their Sociodemographic and Clinical Correlates - doi.org/10.3899/jrheum.190854 November 2020 Featured articles: Cañete, et al: Expert Consensus on a Set of Outcomes to Assess the Effectiveness of Biologic Treatment in Psoriatic Arthritis: The MERECES Study - doi.org/10.3899/jrheum.191056 Aguirre, et al: Using Process Improvement and Systems Redesign to Improve Rheumatology Care Quality in a Safety Net Clinic - doi.org/10.3899/jrheum.190472 Stern, et al: Analysis of Anti-RNA Polymerase III Antibody-positive Systemic Sclerosis and Altered GPATCH2L and CTNND2 Expression in Scleroderma Renal Crisis - doi.org/10.3899/jrheum.190945 Langlois, et al: Rituximab and Cyclophosphamide in Antisynthetase Syndrome–related Interstitial Lung Disease: An Observational Retrospective Study - doi.org/10.3899/jrheum.190505 Moore, et al: Role of Neutrophil Extracellular Traps Regarding Patients at Risk of Increased Disease Activity and Cardiovascular Comorbidity in Systemic Lupus Erythematosus - doi.org/10.3899/jrheum.190875 October 2020 Featured articles: Pappas, et al: Effectiveness of Tocilizumab in Patients with Rheumatoid Arthritis Is Unaffected by Comorbidity Burden or Obesity: Data from a US Registry - doi.org/10.3899/jrheum.190282 van Bentum, et al: The Ankylosing Spondylitis Performance Index: Reliability and Feasibility of an Objective Test for Physical Functioning - doi.org/10.3899/jrheum.191063 Walsh, et al: Measuring Outcomes in Psoriatic Arthritis: Comparing Routine Assessment of Patient Index Data and Psoriatic Arthritis Impact of Disease - doi.org/10.3899/jrheum.190219 Liang, et al: Hemophagocytic Lymphohistiocytosis: Prevalence, Risk Factors, Outcome, and Outcome-related Factors in Adult Idiopathic Inflammatory Myopathies - doi.org/10.3899/jrheum.190542 Kelly, et al: Scope of Outcomes in Trials and Observational Studies of Interventions Targeting Medication Adherence in Rheumatic Conditions: A Systematic Review - doi.org/10.3899/jrheum.190726 September 2020 Featured articles: Kuettel, et al: Pain and Self-reported Swollen Joints Are Main Drivers of Patient-reported Flares in Rheumatoid Arthritis: Results from a 12-month Observational Study - doi.org/10.3899/jrheum.190760 Ben-Shabat, et al: Mortality among Patients with Giant Cell Arteritis: A Large-scale Population-based Cohort Study - doi.org/10.3899/jrheum.190927 Tselios, et al: Advanced Chronic Kidney Disease in Lupus Nephritis: Is Dialysis Inevitable? - doi.org/10.3899/jrheum.191064 Putman, et al: The Quality of Randomized Controlled Trials in High-impact Rheumatology Journals, 1998–2018 - doi.org/10.3899/jrheum.191306 Loef, et al: Health-related Quality of Life in Patients with Hand Osteoarthritis from the General Population and the Outpatient Clinic - doi.org/10.3899/jrheum.190781 July 2020 Featured articles: Harrold, et al: Longterm, Real-world Safety of Adalimumab in Rheumatoid Arthritis: Analysis of a Prospective US-based Registry - doi.org/10.3899/jrheum.190260 Bakewell,et al: Imaging Techniques: Options for the Diagnosis and Monitoring of Treatment of Enthesitis in Psoriatic Arthritis - doi.org/10.3899/jrheum.190512 Hoge, et al: Association of Poverty Income Ratio with Physical Functioning in a Cohort of Patients with Systemic Lupus Erythematosus - doi.org/10.3899/jrheum.190991 Dai, et al: Sleep Quality Is Related to Worsening Knee Pain in Those with Widespread Pain: The Multicenter Osteoarthritis Study - doi.org/10.3899/jrheum.181365 Aydin, etla: The Relationship Between Physical Examination and Ultrasonography of Large Entheses of the Achilles Tendon and Patellar Tendon Origin - doi.org/10.3899/jrheum.190169 back to top June 2020 Featured articles: Sepriano, et al: Adherence to Treat-to-target Management in Rheumatoid Arthritis and Associated Factors: Data from the International RA BIODAM Cohort - doi.org/10.3899/jrheum.190303 Zardin-Moraes, et al: Prevalence of Psoriatic Arthritis Patients Achieving Minimal Disease Activity in Real-world Studies and Randomized Clinical Trials: Systematic Review with Metaanalysis - doi.org/10.3899/jrheum.190677 Desbois, et al: Rituximab-associated Vasculitis Flare: Incidence, Predictors, and Outcome - doi.org/10.3899/jrheum.190076 Bollhalder, et al: Magnetic Resonance Imaging Followup of Temporomandibular Joint Inflammation, Deformation, and Mandibular Growth in Juvenile Idiopathic Arthritis Patients Receiving Systemic Treatment - doi.org/10.3899/jrheum.190168 Colaco, et al: Predictive Utility of Cardiovascular Risk Prediction Algorithms in Inflammatory Rheumatic Diseases: A Systematic Review - doi.org/10.3899/jrheum.190261 May 2020 Featured articles: Skougaard, et al: ELECTOR: eHealth in rheumatology - doi.org/10.3899/jrheum.181362 Lee, et al: Cesarean births in AS - doi.org/10.3899/jrheum.190754 Quinn, et al: Exercise echocardiography in SSc - doi.org/10.3899/jrheum.190226 Gibson, et al: FM assessment screenting tool - doi.org/10.3899/jrheum.190277 Qendro, et al: Immunization in rheumatic diseases - doi.org/10.3899/jrheum.181376 April 2020 Featured articles: Keystone, et al: Primary/secondary nonresponse to anti-TNF - doi.org/10.3899/jrheum.190102 Singh and Cleveland: Insurance, income, and TSA outcomes - doi.org/10.3899/jrheum.190287 Mukwikwi, et al: SLE retinal complications - doi.org/10.3899/jrheum.181102 Rosato, et al: SSc renal parenchymal thickness - doi.org/10.3899/jrheum.190165 Elfishawi, et al: Changes in incident gout - doi.org/10.3899/jrheum.190346 Cron and Chatham: The Rheumatologist’s Role in COVID-19 - doi.org/10.3899/jrheum.200334 Peschken: Possible Consequences of a Shortage of Hydroxychloroquine for Lupus Patients Amid the COVID-19 Pandemic - doi.org/10.3899/jrheum.200395 Putman and Ruderman: Learning from Adversity: Lessons from the COVID-19 Crisis - doi.org/10.3899/jrheum.200411 March 2020 Featured articles: Agca, et al: CV risk in RA - doi.org/10.3899/jrheum.180726 Perruccio, et al: PASDAS and MDA in PsA - doi.org/10.3899/jrheum.181472 Bruschi, et al: NET in SLE/lupus nephritis - doi.org/10.3899/jrheum.181232 Cook, et al: Statins and revision arthroplasty - doi.org/10.3899/jrheum.180574 Singh, et al: Effectiveness of allopurinol in gout - doi.org/10.3899/jrheum.190522 February 2020 Featured articles: Jamal, et al: Adverse events and cancer immunotherapy - doi.org/10.3899/jrheum.190084 Ormseth, et al: Plasma miRNA RA panel - doi.org/10.3899/jrheum.181029 Rostami, et al: AS risk prediction - doi.org/10.3899/jrheum.181209 de Vries-Bouwstra, et al: Recommendation agreement in SSc - doi.org/10.3899/jrheum.181173 Bowes, et al: Automated cartilage segmentation - doi.org/10.3899/jrheum.180541 back to top January 2020 Featured articles: Bechman, et al: Placebo response in RA - doi.org/10.3899/jrheum.190008 Yusuf: Editorial - doi.org/10.3899/jrheum.190900 Walsh, et al: axSpA identification methods - doi.org/10.3899/jrheum.181005 Urowitz, et al: AVE in SLE in decades - doi.org/10.3899/jrheum.180986 Ying, et al: VA SSc stroke risk - doi.org/10.3899/jrheum.181311 Mills, et al: Rheumatic diseases and pregnancy - doi.org/10.3899/jrheum.181067 Author Interviews Q & A: Catherine Bakewell, MD, Sibel Zehra Aydin, MD, Lihi Eder, MD, PhD, and Gurjit S. Kaeley, MBBS, MRCP, RhMSUS Editor-in-Chief Dr. Earl Silverman speaks with Dr. Catherine Bakewell from the Intermountain Healthcare Medical Group Salt Lake Clinic, Dr. Sibel Zehra Aydin from the University of Ottawa, Dr. Lihi Eder at the Women’s College Hospital, and Dr. Gurjit S. Kaeley from the University of Florida about their and their co-authors' review article "Imaging Techniques: Options for the Diagnosis and Monitoring of Treatment of Enthesitis in Psoriatic Arthritis". For the full article: Imaging Techniques: Options for the Diagnosis and Monitoring of Treatment of Enthesitis in Psoriatic Arthritis by Catherine Bakewell, Sibel Zehra Aydin, Veena K. Ranganath, Lihi Eder and Gurjit S. Kaeley. [Read the full transcript.] For the video interview: Viewing Rheum Q & A: Daniel K. White, PT, ScD, MSc Editor-in-Chief Dr. Earl Silverman speaks with Dr. Daniel K. White from the University of Delaware about his and his co-authors' editorial "Walk At Least 10 Minutes a Day for Adults With Knee Osteoarthritis: Recommendation for Minimal Activity During the COVID-19 Pandemic". For the full article: Walk At Least 10 Minutes a Day for Adults With Knee Osteoarthritis: Recommendation for Minimal Activity During the COVID-19 Pandemic by Jason T. Jakiela, Esther J. Waugh, and Daniel K. White. [Read the full transcript.] For the video interview: Viewing Rheum Q & A: Dr. Roberto Caricchio, MD Editor-in-Chief Dr. Earl Silverman speaks with Dr. Roberto Caricchio from Lewis Katz School of Medicine, Temple University about his and his co-author's letter Rheumatologists and Pulmonologists at Temple University Weather the COVID-19 Storm Together. For the full article: Rheumatologists and Pulmonologists at Temple University Weather the COVID-19 Storm Together by Roberto Caricchio and Gerard J. Criner. [Read the full transcript.] For the video interview: Viewing Rheum Q & A: Drs. Michael S. Putman, MD, and Eric M. Ruderman, MD Editor-in-Chief Dr. Earl Silverman speaks with Drs. Michael S. Putman and Eric M. Ruderman from Northwestern University about their editorial Learning from Adversity: Lessons from the COVID-19 Crisis. For the full article: Learning from Adversity: Lessons from the COVID-19 Crisis by Michael S. Putman and Eric M. Ruderman. [Read the full transcript.] For the video interview: Viewing Rheum Q & A: Drs. Rosie Scuccimarri, MD, Evelyn Sutton, MD, and Mary-Ann Fitzcharles, MB, ChB Editor-in-Chief Dr. Earl Silverman speaks with Drs. Rosie Scuccimarri from McGill University, Evelyn Sutton from Dalhousie University, and Mary-Ann Fitzcharles from McGill University about their editorial Hydroxychloroquine: A Potential Ethical Dilemma for Rheumatologists during the COVID-19 Pandemic. For the full article: Hydroxychloroquine: A Potential Ethical Dilemma for Rheumatologists during the COVID-19 Pandemic by Rosie Scuccimarri, Evelyn Sutton, and Mary-Ann Fitzcharles. [Read the full transcript.] For the video interview: Viewing Rheum back to top Audio Abstracts Dr. James T. Rosenbaum For the full article: The Effect of HLA-B27 on Susceptibility and Severity of Covid-19 by James T. Rosenbaum, Hedley Hamilton, Michael H. Weisman, John D. Reveille, Kevin L. Winthrop, and Dongseok Choi. [Read the full transcript.] Dr. Niv Ben-Shabat, BMSc For the full article: Mortality among Patients with Giant Cell Arteritis: A Large-scale Population-based Cohort Study by Niv Ben-Shabat, Shmuel Tiosano, Ora Shovman, Doron Comaneshter, Yehuda Shoenfeld, Arnon D. Cohen and Howard Amital Dr. Gisele Vajgel, MD For the full article: Effect of a Single Apolipoprotein L1 Gene Nephropathy Variant on the Risk of Advanced Lupus Nephritis in Brazilians by Gisele Vajgel, Suelen Cristina Lima, Diego Jeronimo S. Santana, Camila B.L. Oliveira, Denise Maria N. Costa, Pamela J. Hicks, Maria Alina G.M. Cavalcante, Carl D. Langefeld, Lucila Maria Valente, Sergio Crovella, Gianna Mastroianni Kirsztajn, Barry I. Freedman and Paula Sandrin-Garcia Dr. Paula Muilu, MD For the full article: Opioid Use among Patients with Early Inflammatory Arthritides Compared to the General Population by Paula Muilu, Vappu Rantalaiho, Hannu Kautiainen, Lauri Juhani Virta and Kari Puolakka. [Read the full transcript.] Dr. Marie Skougaard, MD For the full article: Patients with Rheumatoid Arthritis Acquire Sustainable Skills for Home Monitoring: A Prospective Dual-country Cohort Study (ELECTOR Clinical Trial I) by Marie Skougaard, Henning Bliddal, Robin Christensen, Karen Ellegaard, Sabrina M. Nielsen, Jakub Zavada, Sabina Oreska, Niels S. Krogh, Christian C. Holm, Merete L. Hetland, Jiri Vencovsky, Henrik Røgind, Peter C. Taylor and Henrik Gudbergsen. [Read the full transcript.] back to top Techniques Dr. Edward C. Keystone, MD, FRCP(C) For the full article: The Dorsal 4-finger Technique: A Novel Method to Examine Metacarpophalangeal Joints in Patients with Rheumatoid Arthritis by Mohammed A. Omair, Pooneh Akhavan, Ali Naraghi, Shikha Mittoo, Juan Xiong, Deborah Weber, Daming Lin, Melissa Weber, and Edward C. Keystone. [Read the full transcript.] back to top Contents Author Interviews Audio Abstracts Techniques Editor's Picks Archives Author Interviews Audio Abstracts Editor's Picks 2019 Editor's Picks 2018

|
Scooped by
Gilbert C FAURE
April 3, 2022 4:55 AM
|
Published in Sage Journals - Link to article Tendons and ligaments are essential connective tissues that connect the muscle and bone. Their recovery from injuries is known to be poor, highlighting the crucial need for an effective therapy.

|
Scooped by
Gilbert C FAURE
July 12, 2021 1:45 PM
|
With durable cancer responses, genetically modified cell therapies are being implemented in various cancers. However, these immune effector cell therapies can cause toxicities, including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).

|
Scooped by
Gilbert C FAURE
March 19, 2021 8:29 AM
|
We've published an updated guideline on the treatment of giant cell arteritis (GCA), a condition diagnosed in around 2,500 people in the UK every year. It’s a serious, autoimmune condition in which blood vessels become inflamed and can restrict blood flow. We spoke to guideline co-lead, Dr Sarah Mackie, about what's changed and how the guideline improves care for patients across the UK. GCA affects the blood supply to the scalp, jaw muscles or the back of the eye and is treated with high-dose glucocorticoids (steroids). If left untreated, it can lead to blindness or stroke. Fortunately, in most cases GCA is caught in time, but it's thought that up to one in five patients may experience a degree of permanent loss of vision from the disease. This means early diagnosis and prompt treatment is essential. Our updated guideline aims to ensure clinicians have the latest information about the diagnosis and treatment of the condition. It brings the latest peer-reviewed evidence up-to-date and supports clinicians in providing the best treatment for people with GCA. Dr Sarah Mackie, Associate Clinical Professor in Vascular Rheumatology at the University of Leeds, co-led the development of the guideline, working with over 35 national and international experts in the field, including rheumatologists, GPs, ophthalmologists and patients. It involved a rigorous process using a framework for evidence appraisal called GRADE, coupled with BSR's guidelines protocol, which is endorsed by NICE. Dr Mackie says: “The way patients with suspected GCA have been assessed and treated is variable across the UK. Giant cell arteritis is time-critical; a delay in starting high-dose steroid treatment can cause blindness, but this same treatment can cause serious side-effects, so this is not a matter to be taken lightly. We recommend all patients are referred to a specialist who can see them promptly – on the same working day if possible and in all cases within three working days.” There have been major developments in the treatment of giant cell arteritis since the last guideline was produced in 2010, particularly with imaging and biologic therapy. Dr Mackie continues: “This guideline provides a coherent statement of what is the latest best practice. It also means that care can be standardised for all patients.” The two major new areas of evidence are: Diagnostic imaging: The guideline recommends a sequence of tests, including if possible, the use of vascular ultrasound before a temporal artery biopsy for a faster, accurate diagnosis. It can often be useful to do both an ultrasound and a temporal artery biopsy; the guidelines explain when to do this. Ultrasound for diagnosis for GCA is quite a new test and not all hospitals have access to this yet. Biologic therapy: Since the previous guidelines, the drug tocilizumab has been licensed for GCA, prescribed alongside steroids for patients who have relapsed, as well as for the small minority who do not respond to initial steroid treatment. Trials suggest adding tocilizumab can reduce the risk of further relapse and so lessen patients’ overall exposure to steroids. The guideline reviews this latest evidence. The guideline also includes practical information for clinicians including what symptoms to check, what tests to do, steroid dosing and care pathways. Charities such as PMRGCA UK, which was involved in the development of the updated guideline, welcomes this development. Humphrey Hodgson, Chair of Trustees for PMRGCA UK, says it's vital the guideline is rolled out across the UK: “These new guidelines have the power, if implemented fully, to transform the diagnosis and treatment of giant cell arteritis. Too often our charity learns of cases of people losing all or some of their sight needlessly because diagnosis was delayed, or the wrong treatment given. People with GCA have the right to fast-track treatment to save their sight just as fast-track treatment has transformed outcomes for those who have strokes.” Dr Mackie concludes: “These guidelines help clinicians who are trying to improve their local service for patients with suspected GCA. The standardised approach to care outlined in the guidelines supports clinicians in conversations with their managers about developing business cases for investment in this area. By talking about the guideline and using it, we'll help raise the profile of this condition and drive forward best practice.”

|
Scooped by
Gilbert C FAURE
February 2, 2021 3:12 AM
|
Strategies for delivering nucleic acids into damaged and diseased tissues have been divided into two major areas: viral and non-viral gene therapy. In this mini-review article we discuss the application of gene therapy for the treatment of osteoarthritis (OA), one of the most common forms of arthrit …

|
Scooped by
Gilbert C FAURE
November 28, 2020 4:42 AM
|
Research ArticleCell biologyMetabolism Open Access | 10.1172/jci.insight.139032 Pathogenic, glycolytic PD-1+ B cells accumulate in the hypoxic RA joint Achilleas Floudas,1 Nuno Neto,2,3 Viviana Marzaioli,1,4 Kieran Murray,4 Barry Moran,5 Michael G. Monaghan,2,3 Candice Low,4 Ronan H. Mullan,6 Navin Rao,7 Vinod Krishna,7 Sunil Nagpal,7 Douglas J. Veale,4 and Ursula Fearon1,4 Published November 5, 2020 - More info View PDF Abstract While autoantibodies are used in the diagnosis of rheumatoid arthritis (RA), the function of B cells in the inflamed joint remains elusive. Extensive flow cytometric characterization and SPICE algorithm analyses of single-cell synovial tissue from patients with RA revealed the accumulation of switched and double-negative memory programmed death-1 receptor–expressing (PD-1–expressing) B cells at the site of inflammation. Accumulation of memory B cells was mediated by CXCR3, evident by the observed increase in CXCR3-expressing synovial B cells compared with the periphery, differential regulation by key synovial cytokines, and restricted B cell invasion demonstrated in response to CXCR3 blockade. Notably, under 3% O2 hypoxic conditions that mimic the joint microenvironment, RA B cells maintained marked expression of MMP-9, TNF, and IL-6, with PD-1+ B cells demonstrating higher expression of CXCR3, CD80, CD86, IL-1β, and GM-CSF than their PD-1– counterparts. Finally, following functional analysis and flow cell sorting of RA PD-1+ versus PD-1– B cells, we demonstrate, using RNA-Seq and emerging fluorescence lifetime imaging microscopy of cellular NAD, a significant shift in metabolism of RA PD-1+ B cells toward glycolysis, associated with an increased transcriptional signature of key cytokines and chemokines that are strongly implicated in RA pathogenesis. Our data support the targeting of pathogenic PD-1+ B cells in RA as a focused, novel therapeutic option. Graphical Abstract Introduction Rheumatoid arthritis (RA) is the most common inflammatory arthropathy and is characterized primarily by the presence of circulating autoantibodies, first described in the 1940s. It often has a progressive and debilitating course, with significant impact on the patient’s quality of life. Despite the long-known association with autoantibodies, knowledge of the role of B cells and their potential direct contribution to disease pathogenesis in RA is limited (1). The recent observation of increased expression of programmed death-1 receptor (PD-1) in subjects who are autoantibody positive, even before they develop RA, suggests this is a primary immune dysregulation in the disease (2). Until recently, the main focus of PD-1 expression and therapeutic targeting of this pathway has been on T cells in the immune response of patients with cancer, which interestingly has led some patients to develop autoimmune diseases, including arthritis (3). Synovial accumulation of B cells correlates with increased radiographic scores and T cell activation in patients with RA; consequently, B cell–targeting therapies have demonstrated promising results for the treatment of RA, with rituximab (anti-CD20) showing significant efficacy and amelioration of disease progression in patients naive to methotrexate and those with incomplete responses to a TNF inhibitor (4–7). B cell depletion leads to significant but limited reduction in anti-citrullinated protein antibodies (ACPAs) and rheumatoid factor (RF), with studies showing clinical benefit for both autoantibody-positive and autoantibody-negative patients with RA (8, 9). While the exact mechanism leading to disease amelioration following B cell depletion is not fully elucidated, at-risk individuals who received a single dose of rituximab had a significant delay in disease onset that did not correlate with a reduction in IgG-RF or ACPA (10, 11). These studies highlight a key role for B cells at an early stage of disease pathogenesis in RA, in addition to their capacity to produce potentially autoreactive antibodies. Several aspects of B cell depletion remain poorly understood, which if uncovered, could exert a significant influence on therapeutic outcomes. Recent studies show a population of CD20+ T cells is subject to rituximab-mediated depletion (12). These cells express high levels of proinflammatory cytokines IL-17, TNF-α, and IFN-γ, and their potential contribution to the therapeutic effect of rituximab needs to be taken into consideration (13). Plasmablasts and plasma cells do not express CD20 and therefore are not affected by rituximab-mediated B cell depletion. A potential exception, however, has been described in a mouse model of inflammatory arthritis, where short-lived plasma cells residing in the spleen and secondary lymph nodes were shown to express CD20 and thus subject to depletion (14). These cells were preferentially autoreactive, highlighting that although overall antibody titers might remain unchanged following B cell depletion, certain antibody antigen specificities might be preferentially lost. Another complication that arises with current B cell–depleting strategies is the composition and immunological effect of B cell repopulation and the influence that may have on subsequent immune responses. Studies on B cell repopulation, following B cell depletion, have shown that returning B cells are primarily naive, immature, and enriched for IL-10–expressing cells (15, 16). Therefore, we hypothesized that there are missed opportunities for targeted therapeutic intervention that could potentially minimize off-target effects of B cell depletion by focusing either on the migration of memory B cells to the inflamed RA joint or on specific pathogenic B cell subpopulations, leaving the majority of the B cell pool intact. We have therefore performed extensive characterization of B cell subpopulations in the peripheral blood, synovial fluid, and synovial tissue of patients with early RA and have identified CXCR3 as a major contributor of memory B cell migration to the synovial tissue in RA. Importantly, a subpopulation of CXCR3+ B cells constitutively expresses PD-1. PD-1+ RA B cells maintained higher T cell costimulatory capacity and proinflammatory cytokine production than their PD-1– counterparts, under normoxic and hypoxic conditions that more closely resembled the environment of the inflamed RA joint. PD-1+ B cells accumulated at the site of inflammation in RA, showed increased activation of key metabolic pathways, including AKT/mTOR/S6 signaling pathway activity; and were dependent on STAT3 activation and glucose uptake. Using emerging noninvasive fluorescent lifetime imaging microscopy (FLIM) metabolic imaging technique and RNA-Seq, we show that these RA PD-1+ B cells are hyperactive and demonstrate increased glycolytic capacity. Results Accumulation of double-negative and switched memory B cells at the synovial tissue of RA patients. Multiparametric flow cytometric analysis for the identification of naive (IgD+CD27–), CD27+ memory, non–switched memory (IgD+CD27+), switched memory (IgD–CD27+), double-negative memory (IgD–CD27–), transitional (CD24hiCD38hi), and IgM-only memory (IgD–CD27+IgM+) B cell subpopulations and plasma cells (CD138+CD27hi) was performed (Figure 1A). Unexpectedly, a highly significant (P = 0.0022) increased frequency of naive B cells in the peripheral blood of healthy controls (HCs) compared with patients with RA was observed (Figure 1B). This difference was coupled with a significantly (P = 0.009) reduced frequency of CD27+ memory B cells in RA compared with HC that was similarly distributed between switched (P = 0.02) and non–switched memory (P = 0.04) B cells (Figure 1B). Although no significant differences were observed in the frequency of IgM-only memory B cells or plasma cells between RA and HC, a significant reduction in the frequency of transitional CD24hiCD38hi enriched for IL-10–producing B cells was observed in RA patient compared with HC peripheral blood (P = 0.04) (Figure 1B). Analysis of T cell costimulatory molecules of RA patient and HC peripheral blood B cells revealed no significant differences in the expression of CD80, CD86, HLA-DR, or CD40 (Figure 1C). Figure 1 RA patient peripheral blood B cell subpopulation distribution. (A) Representative flow cytometric analysis gating strategy for the identification of naive (IgD+CD27–), non-switched memory (IgD+CD27+), switched memory (IgD–CD27+), and double-negative memory (IgD–CD27–) B cells (over 20 independent experiments performed). DN, double-negative; L/D, LIVE/DEAD stain. (B) Frequency of the indicated B cell populations in the PBMCs of HC (n = 9–13) and RA patients (n = 18–30). Data are presented as mean ± SEM. Each symbol represents an individual sample. Statistical analysis was performed by using standard Student’s t test. *P < 0.05. (C) MFI values for the expression of CD40, CD86, CD80, and MHCII (HLA-DR) for HC and RA patient peripheral blood CD19+CD20+ B cells. Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. Data are presented as mean ± SEM; each symbol represents an individual sample. Statistical analysis was performed by using 1-way ANOVA with Tukey’s multiple-comparisons test. *P < 0.05, **P < 0.01. We then performed an extensive characterization of B cell subpopulations in RA patient synovial fluid (SF) and enzymatically and mechanically digested synovial tissue. While the frequency of CD19+CD20+/– cells was significantly lower in RA patient SF (P = 0.0005) and synovial tissue (P = 0.02) compared with peripheral blood, the subpopulation distribution was markedly different (Supplemental Figure 1 and Supplemental Figure 2; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.139032DS1). Interestingly, a significant reduction in naive (IgD+CD27–) B cells and a dramatic increase in the frequency of switched memory and double-negative memory B cells in RA SF (P < 0.0001 for all) and synovial tissue (P < 0.0001, P = 0.01, P = 0.0002, respectively) compared with peripheral blood was observed (Figure 2, A–C). Figure 2 Synovial tissue accumulation of DN and switched memory B cells in RA. (A) Representative plots of CD19+CD20+ B cells for the expression of IgD and CD27 in the peripheral blood, SF, and synovial tissue (at least 5 independent experiments performed). (B) Average subpopulation distribution of peripheral blood HC and RA patient B cells and RA patient SF and synovial tissue B cells. (C) Frequency of the indicated B cell populations in the periphery (n = 20), SF (n = 12), and synovial tissue (n = 6) of RA patients. Data are presented as mean ± SEM. Each symbol represents an individual sample. Statistical analysis was performed by using 1-way ANOVA with Tukey’s multiple-comparisons test. **P < 0.01, ***P < 0.001. CXCR3 is an important mediator for the accumulation of memory B cells to the site of inflammation in RA. We then examined the potential chemokine receptors involved in the accumulation of switched memory and DN memory B cells at the inflamed joint in RA. An extensive characterization of chemokine receptors was performed by flow cytometric analysis of the peripheral blood, SF, and synovial tissue B cells, including CXCR3, CXCR5, CCR6, and CCR7 (Figure 3, A and B). Chemokine receptor expression pattern differences were observed between HC and RA patient–derived peripheral blood. Such differences could potentially be utilized as novel diagnostic tools (Figure 3A). A significant increase in the expression of the chemokine receptor CXCR3 was evident for RA SF (P < 0.0001) and synovial tissue (P = 0.0007) B cells compared with peripheral blood B cells (Figure 3, B and C). A relatively small population (approximately 20%) of peripheral blood B cells expressed CXCR3, in comparison with approximately 80% positivity observed at the site of inflammation (Figure 3C). Peripheral blood CXCR3+ B cells belonged primarily to the switched memory and DN memory B cell subpopulations, therefore closely resembling the frequency of these cells at the RA synovial tissue (Figure 3, D and F), suggesting that CXCR3 is an important mediator of memory B cell accumulation from the periphery to the site of inflammation in RA. The local microenvironment of the inflamed joint could further contribute to B cell CXCR3 expression since CXCR3 is inducible by TNF and IFN-γ, 2 common proinflammatory cytokines of the joint microenvironment, but suppressed by IL-4 (Figure 3E). RA patient–derived B cells had the capacity to invade in response to RA synovial biopsy-conditioned media; however, upon treatment with the CXCR3 antagonist AMG487, B cell invasion was significantly reduced (P = 0.03) (Figure 3F). Importantly, there was a significant inverse correlation (r = –0.6, P = 0.047) between the peripheral blood frequency of CXCR3+ B cells and DAS28-CRP in patients with RA, potentially due to increased migration of CXCR3-expressing B cells to the site of inflammation in patients with higher disease severity, therefore further highlighting the importance of CXCR3 expression for the migration of B cells to the inflamed joint and disease progression (Figure 3G). Interestingly, 6 months to 1 year following rituximab-mediated B cell depletion, the returning B cells were primarily transitional B cells (P < 0.0001) expressing high levels of CD5 (associated with regulatory B cell function) (P = 0.0042) and CXCR3 (P < 0.0001) compared with B cells of patients who had not received B cell depletion therapy (Supplemental Figure 3). These studies highlight an important role for CXCR3 in the accumulation of memory B cells from the periphery to the inflamed synovial tissue. The potential for CXCR3-mediated trafficking of transitional B cells to the site of inflammation after B cell depletion therapy and any possible contribution to disease amelioration warrant further examination. Figure 3 Involvement of CXCR3 in the migration of peripheral blood memory B cells to the synovial tissue. (A) SPICE algorithm flow cytometric analysis of peripheral blood and synovial tissue RA patient B cell expression of the chemokine receptors CXCR3, CXCR5, CCR6, and CCR7. (B) Representative gating followed for the flow cytometric analysis and identification of CXCR3-expressing peripheral blood, SF, and synovial tissue B cells (at least 6 independent experiments were performed). FMO, fluorescence minus one. (C) Frequency of RA patient CXCR3-expressing B cells in the periphery (n = 12), SF (n = 7), and synovial tissue (n = 6). Data are presented as mean ± SEM. Statistical analysis was performed by using 1-way ANOVA with Tukey’s multiple-comparisons test. **P < 0.01, ***P < 0.001. (D) Representative flow cytometric analysis plots and frequency of RA patient switched memory and DN memory B cells within the CXCR3+ peripheral blood B cell compartment (n = 4). Data are presented as mean ± SEM. Ordinary 2-way ANOVA with Holm-Šidák multiple-comparisons test was performed. (E) Effect of CXCR3 expression change following incubation of isolated RA patient–derived peripheral blood B cells with the indicated cytokines (n = 6/group). (F) Representative flow cytometric analysis and CD19+/counting bead ratio for of invading B cells toward cRPMI (control), RA synovial biopsy-conditioned media (SP), or RA synovial biopsy-conditioned media following treatment of the B cells with the CXCR3 small molecule antagonist AMG487 (n = 7/group, 3 independent experiments); 1-way ANOVA with Tukey’s multiple-comparisons test; *P < 0.05. Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. (G) Linear regression analysis between the frequency of RA patient peripheral blood CXCR3+ B cells and DAS28; n = 11; each symbol represents an individual sample. Spearman r correlation analysis was performed. RA patient–derived B cells express high levels of proinflammatory cytokines under hypoxic conditions, mimicking the environment of the inflamed RA joint. Previous studies have shown a positive correlation between hypoxia and cellular infiltration of the RA synovial tissue (17, 18). We therefore examined the effect of hypoxic conditions that mimic the microenvironment of the inflamed joint on B cell activation and cytokine production. RA patient– or HC-derived B cells were isolated and cultured under atmospheric oxygen levels (normoxia) or 3% O2 (hypoxia), which is the previously estimated in vivo average oxygen concentration in the inflamed joints of RA patients (17, 18). The expression of TNF, IL-6, and IL-1β by RA- and HC-derived B cells stimulated under normoxic and hypoxic conditions was examined. Under normoxic conditions, there was increased (P = 0.0047) IL-1β production but not TNF or IL-6 by RA-derived, compared with HC-derived, B cells when stimulated via the B cell receptor (BCR) with additional TLR stimulation (Figure 4, A–C). Under hypoxic conditions that mimic the microenvironment of the inflamed joint, however, RA-derived B cells secreted significantly higher IL-6 and TNF when stimulated through the BCR (P = 0.0003, P = 0.007, respectively) with or without additional TLR stimulation (P = 0.0007, P = 0.03, respectively) (Figure 4, A–C). In addition to the increased proinflammatory cytokine production, under hypoxic conditions, RA patient–derived B cells showed significantly higher expression of MMP-9 (P = 0.005), which is indicative of increased invasive capacity compared with HC-derived B cells (Figure 4D). These data demonstrate the importance of oxygen availability for B cell stimulation and cytokine production and raise important considerations for the interpretation of in vitro data performed under atmospheric O2 conditions. Figure 4 The effect of hypoxia on RA patient–derived B cells. (A) Schematic representation of peripheral blood B cell isolation and stimulation in vitro under normoxic or hypoxic conditions. aCD40, anti-CD40. (B) ELISA for the assessment of IL-6 and TNF-α concentration in HC- or RA patient–derived B cell cultures following stimulation as indicated under normoxic or hypoxic conditions (n = 4–6/group). Ordinary 2-way ANOVA with Holm-Šidák multiple-comparisons test was performed. *P < 0.05, **P < 0.01, ***P < 0.001. (C) Representative flow cytometric analysis plots and frequency of IL-1β–expressing HC- or RA patient–derived B cells following in vitro stimulation under the designated conditions (n = 3/group, 2 independent experiments). Ordinary 2-way ANOVA with Holm-Šidák multiple-comparisons test was performed. **P < 0.01. (D) Fold expression change of RA patient–derived B cell MMP-9 expression compared with HC-derived B cells following stimulation under hypoxic conditions (n = 5/group). Statistical analysis was performed by using paired standard Student’s t test. *P < 0.05, ***P < 0.001. All data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. Activated PD-1+ B cells accumulate at the site of inflammation in RA. Following in vitro stimulation by BCR-mediated signals, RA patient–derived B cells showed marked upregulation of PD-1 expression under normoxic or hypoxic conditions (Figure 5A). Earlier in this study, we demonstrated the importance of CXCR3 for the accumulation of memory B cells to the site of inflammation in RA. Importantly, the majority of CXCR3-expressing B cells following activation in vitro constitutively expressed PD-1 (Figure 5B). PD-1–expressing B cells constitute a rare (~2% of CD19+) population of cells in the periphery; however, there was significant accumulation of these cells in the SF (P = 0.0002) and synovial tissue (P = 0.005) in RA (Figure 5C). Immunofluorescence analysis of RA patient synovial tissue biopsies showed preferential accumulation of CD19+PD-1+ cells in tertiary lymphoid-like structures (Supplemental Figure 4). RA patient–derived PD-1–expressing B cells had higher expression of CD86 and CD80 compared with their PD-1– counterparts under normoxic (P = 0.0026, P = 0.001, respectively) and hypoxic (P = 0.0015, P < 0.0001, respectively) conditions (Figure 5D). Importantly, PD-1+ RA patient–derived B cells maintained significantly higher expression of IL-1β under normoxic (P = 0.0031) conditions and GM-CSF under normoxic and hypoxic conditions (P = 0.0003, P = 0.013, respectively) compared with PD-1– counterparts (Figure 5E). Figure 5 Identification of synovial PD-1+ B cells in RA. (A) Representative flow cytometric analysis and cumulative data for the identification of PD-1+ RA patient–derived B cells following in vitro stimulation under the indicated conditions (n = 3–6/group, 3 independent experiments). Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. (B) Frequency of CXCR3 expression by PD-1– and PD-1+ RA patient–derived B cells under the indicated conditions. n = 5–7/group and n = 3 for aCD40. Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. Ordinary 2-way ANOVA with Holm-Šidák multiple-comparisons test was performed. *P < 0.05, **P < 0.01. (C) Frequency of RA patient peripheral blood (PBMC), SF (SFMC), and synovial tissue (Bio) PD-1–expressing CD3+ T cells and CD19+ B cells. n = 3–7/group. Each symbol represents an individual sample. Data are presented as mean ± SEM. Ordinary 2-way ANOVA with Holm-Šidák multiple-comparisons test was performed. **P < 0.01, ***P < 0.001. (D) Representative flow cytometric analysis plots and frequency of CD80 and CD86 expression by RA patient–derived B cells following in vitro stimulation (aCD40+aBCR+CpG) under normoxic or hypoxic conditions (n = 8, 3 independent experiments). Each symbol represents an individual sample. Paired Student’s t test was performed. **P < 0.01, ***P < 0.001. (E) Frequency of IL-1β– and GM-CSF–expressing PD-1– and PD-1+ RA patient–derived B cells following stimulation under the indicated conditions (n = 6, 3 independent experiments). Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. Statistical analysis was performed by using paired standard Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. Previous studies have shown a potential immunoregulatory effect of PD-1+ B cells in patients with thyroid cancer because of an increased expression of PD-L1 by these cells (19). PD-1+ RA patient–derived B cells stimulated in vitro showed similar PD-L1 expression levels compared with matched PD-1– B cells (Supplemental Figure 5). PD-1+ RA patient B cells are dependent on glucose uptake and STAT3 activation. PD-1 expression is dependent on BCR-mediated signals, with TLR9 activation enhancing that effect. Because of the dependency of PD-1 on BCR signaling, the activation of AKT, a downstream kinase of the BCR signaling cascade, was analyzed under normoxic and hypoxic conditions. RA patient–derived PD-1+ B cells expressed significantly (P = 0.011 normoxia, P = 0.022 hypoxia) higher levels of activated AKT compared with PD-1– counterparts (Figure 6A) (20). AKT is a key regulator of mTOR. PD-1+ B cells showed higher activation of mTOR compared with PD-1– B cells (Figure 6A) (21). To assess if the increased phosphorylation of mTOR translates to higher downstream activity, the phosphorylation of the ribosomal protein S6, a target of the mTOR pathway, was assessed. In agreement with the increased AKT/mTOR activity, PD-1+ B cells showed significantly (P = 0.023 normoxia, P = 0.031 hypoxia) higher S6 phosphorylation compared with PD-1– B cells (Figure 6A). Figure 6 PD-1+ RA patient B cells are dependent on glycolysis. (A) Representative flow cytometric analysis histograms and cumulative MFI of RA patient peripheral blood–derived PD-1– and PD-1+ B cell expression of phosphorylated AKT, mTOR, and S6 following stimulation (aCD40+aBCR+CpG) under normoxic (21% O2) and hypoxic (3% O2) conditions (n = 6/group, 3 independent experiments). Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. (B) Representative flow cytometric analysis plots and MFI of RA patient–derived PD-1– and PD-1+ B cell expression of GLUT1 and STAT3 phosphorylation (pSTAT3) following stimulation under the indicated conditions (n = 4/group, 2 independent experiments). Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. (C) Representative flow cytometric analysis histograms of glucose analog 2-NBDG uptake by RA patient–derived PD-1– and PD-1+ B cells under the indicated conditions, (n = 5/group, 2 independent experiments). Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. Ordinary 2-way ANOVA with Holm-Šidák multiple-comparisons test was performed. *P < 0.05, **P < 0.01. (D) Representative flow cytometric analysis plots of PD-1 expression by RA patient–derived B cells following stimulation in the presence of glucose analog 2DG (n = 4, 2 independent experiments). (E) Frequency of PD-1 B cells following incubation with STAT3 small molecule inhibitor Stattic (n = 3). Data are presented as mean ± SEM. Ordinary 2-way ANOVA with Holm-Šidák multiple-comparisons test was performed. **P < 0.01. (F) Effect of PD-1/PD-L1 engagement on PD-1+ RA patient–derived B cell expression of CD80 and CD86 and phosphorylation of AKT, mTOR, and S6 under normoxic or hypoxic conditions (n = 9). Data are represented as a box-and-whisker plot, with bounds from 25th to 75th percentile, median line, and whiskers ranging from 5th to 95th percentile. Statistical analysis was performed by using paired standard Student’s t test. The AKT/mTOR pathway has previously been shown to control glucose uptake and metabolism; therefore, we examined glucose transporter 1 (GLUT1) expression in RA patient PD-1+ and PD-1– B cells (22, 23). BCR engagement led to increased B cell GLUT1 expression that correlates with STAT3 phosphorylation, indicating a potential association between BCR signaling strength and GLUT1 upregulation, while additional TLR9-dependent signals enhanced the BCR-mediated effect (Figure 6B). PD-1+ B cells had significantly (P = 0.002 and P = 0.015, respectively) higher expression of GLUT1 and phosphorylation of STAT3 (P = 0.014 and P = 0.021, respectively) than PD-1– B cells under normoxic and hypoxic conditions (Figure 6B). The increased expression of GLUT1 led to significantly increased glucose uptake of PD-1+ compared with PD-1– B cells, under normoxic (P = 0.002) and hypoxic (P = 0.015) conditions, as assessed by the incorporation of the fluorescent glucose analog 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) (Figure 6C). Additional indication that PD-1–expressing RA patient B cells rely on glycolysis is the increased mitochondrial mass of these cells under normoxic and hypoxic conditions compared with their PD-1– counterparts (Supplemental Figure 6). Deprivation of glucose by addition of the glucose analog 2-deoxyglucose (2DG) that fails to undergo glycolysis led to the elimination of PD-1–expressing B cells under in vitro stimulation conditions (Figure 6D). PD-1+ B cells had higher activation of STAT3 compared with PD-1– B cells; therefore, we inhibited STAT3 activation using a small molecular weight inhibitor, Stattic (24). STAT3 inhibition led to complete loss of PD-1+ B cells under normoxic and hypoxic conditions (P = 0.0014, P = 0.004, respectively) (Figure 6E). PD-1/PD-L1 interactions have been proposed to dampen down the BCR-mediated downstream signaling (25). Plate-bound recombinant PD-L1 was utilized to assess the effect of PD-1/PD-L1 engagement on the activation and metabolic status of PD-1+ B cells. PD-L1 did not result in decrease of CD80 and CD86 expression or AKT, mTOR, and S6 activation under normoxic or hypoxic conditions (Figure 6F). Altered cytokine, antigen presenting, and glycolysis gene signatures in ex vivo PD-1+ RA B cells. RNA-Seq analysis of ex vivo patient-derived B cells sorted on the basis of PD-1 revealed altered expression of approximately 900 genes between PD-1+ B cells and their PD-1– counterparts (Figure 7A). Principal components analysis (PCA) of PD-1+ and PD-1– B cells showed a separation between the 2 populations (Figure 7B). Proinflammatory cytokine gene expression for cytokines previously implicated in RA pathogenesis, namely TNFA, IL6, IL1B, and IL32, was significantly (P < 0.001) increased in PD-1+ B cells compared with PD-1– B cells while the immunomodulatory IL24 was significantly (P < 0.001) decreased (Figure 7C). Alterations were also observed in chemokine expression and genes involved in B cell maturation and activation, with PD-1+ B cells adopting an overall more activated profile than PD-1– B cells (Figure 7C). Several genes involved in glycolysis were significantly upregulated in PD-1+ B cells in contrast to PD-1– B cells, with pathway analysis showing enrichment in the glycolysis (P = 4.3 × 10–9), gluconeogenesis (P = 8.7 × 10–7), and oxidative phosphorylation (P = 4 × 10–40) pathways (Figure 7C, Supplemental Figure 7). To complement this analysis, a gene set enrichment analysis (GSEA) was performed on the differentially expressed genes ranked by their fold change values, using the Hallmark gene signature from the Broad Institute’s Molecular Signatures Database. This analysis showed an enrichment in the glycolysis gene signature (normalized enrichment score [NES] = 1.37, FDR = 0.222) and a stronger enrichment in genes corresponding to inflammatory response (NES = 1.77, FDR = 0.008) and TNF-α signaling through NF-κB (NES = 1.60, FDR = 0.018). Figure 7 Differential gene expression of ex vivo RA patient PD-1+ and PD-1– B cells. (A) Differential gene expression volcano plot of flow sorted, ex vivo RA patient–derived PD-1+ compared with PD-1– B cells. Red-colored genes show significant differential expression between the 2 groups. (B) PCA analysis of flow sorted, ex vivo RA patient PD-1+ and matched PD-1– B cells. Each point represents an independent sample. (C) Heatmap of RNA-Seq expression Z-scores for selected differentially regulated genes between PD-1+ and PD-1– B cells. Each column represents an individual sample. RA patient PD-1+ B cells are more glycolytic than their PD-1– counterparts. The data presented herein suggest an increased glycolytic capacity and glucose dependency of PD-1+ compared with PD-1– RA patient–derived B cells. In order to directly assess whether PD-1+ B cells are more reliant on either glycolysis or oxidative phosphorylation (OXPHOS), FLIM was utilized. FLIM is based on the principle of endogenous fluorescence molecules such as NAD and as a result requires no staining, fixation, or other type of processing of the target cells (26). FLIM’s capacity to distinguish between bound and unbound NAD is based on the self-quenching ability of NAD. In unbound NAD the nicotinamide and adenine rings are in close proximity, and the fluorescent signal decay following excitation is approximately 0.4 ns; however, NAD in its bound form has a significantly lower signal decay in the range of 2 ns because of stretching of the molecule, leading to longer distance between the nicotinamide and adenine rings and reduced self-quenching (26, 27). Importantly, higher unbound to bound NAD ratio is directly proportionate to the cell’s glycolytic versus oxidative metabolic capacity. Visualization of NAD by FLIM offers direct evidence of the cell’s metabolic state. FLIM analysis of RA patient–derived PD-1+ sorted B cells revealed a significant (P = 0.022) preference for glycolysis compared with matched PD-1– B cells (Figure 8, A and B). Figure 8 FLIM analysis of PD-1 B cell metabolic profile. (A) Representative multiphoton microscopy FLIM analysis of flow sorted RA patient–derived PD-1– and PD-1+ B cells (4 independent experiments were performed). (B) Average PD-1– and PD-1+ B cell emission lifetime (τavg) of NAD following excitation. n = 4/group. Data are presented as mean ± SEM. A reduction in τavg is reflected in an increase in free NAD and therefore increased glycolysis. Statistical analysis was performed by using paired standard Student’s t test. *P < 0.05. Discussion B cell depletion therapy has been efficacious for the treatment of patients with RA. However, opportunities for more targeted therapeutic intervention that can minimize potential side effects should be explored. Herein, we demonstrate a role for CXCR3 in the accumulation of switched and DN memory B cells at the site of inflammation in RA. Peripheral blood CXCR3-expressing B cells closely mirrored the B cell subpopulation distribution in the inflamed RA joint with overrepresentation of switched memory and DN memory B cells. A negative correlation between the frequency of CXCR3-expressing B cells and disease activity in RA patients, potentially because of increased migration of peripheral blood CXCR3-expressing B cells to the site of inflammation in RA patients with increased disease severity, further reinforces the contribution of CXCR3 in the migration of activated memory B cells and raises the possibility for early therapeutic intervention by inhibiting the CXCR3-mediated synovial migration of memory B cells. We have previously demonstrated that the RA joint is hypoxic with an average oxygen level of 3% and a positive correlation between hypoxia and synovial tissue cellular infiltration (17, 18). The effect of hypoxia on B cell function has not been fully elucidated; therefore, we examined the activation and costimulatory potential of RA patient–derived B cells, stimulated with physiologically relevant conditions under hypoxia, and identified a superior capacity of these cells to maintain proinflammatory cytokine production in a hypoxic environment recapitulating the inflamed joint. While the effect of hypoxia on RA B cell proinflammatory cytokine production was limited, with RA patient–derived B cells maintaining their capacity to produce proinflammatory cytokines, hypoxic conditions led to a reduction of proinflammatory cytokine production by HC-derived B cells. Therefore, hypoxia exacerbated the differences between HC- and RA patient–derived B cells. Additionally, RA patient–derived B cells cultured under hypoxic conditions expressed high levels of MMP-9 compared with HC-derived B cells; B cell MMP-9 expression has previously been correlated with clinical relapse in patients with multiple sclerosis and the capacity of B cells to invade the blood-brain barrier (28). In this study we identified a population of CXCR3hiPD-1+ B cells that preferentially accumulated in the synovial tissue in RA, as opposed to the peripheral blood, and were characterized by a markedly increased capacity for costimulation and proinflammatory cytokine production. PD-1+ B cells showed a strong dependency on glucose uptake and STAT3 activation and, based on direct visualization of bound and unbound forms of NAD by the potentially novel FLIM, were more glycolytic than their PD-1– counterparts. Altered gene expression in over 900 genes was observed between PD-1+ and PD-1– ex vivo patient B cells, with genes involved in B cell activation and proinflammatory cytokine production being upregulated in PD-1+ B cells compared with PD-1– B cells. Additionally the glycolysis, gluconeogenesis, and oxidative phosphorylation pathways were enriched in PD-1–expressing B cells. Several studies have previously demonstrated the capacity of B cells to express the T cell–associated coinhibitory factor PD-1. There is, however, a paucity of information on the functional effects of B cell PD-1 expression. A proposed mechanism of action for B cell PD-1 expression is the dampening of BCR-mediated downstream signaling, leading to decreased B cell activation and cytokine production (25, 29). Recent studies identify potentially tumorigenic B cells that express PD-1 and promote immune system regulation and tumor survival; however, there are discrepancies regarding the suggested mechanisms that these cells employ to exert their immunosuppressive effects. A study in patients with hepatoma shows PD-1+ B cell IL-10–dependent immune suppression of antitumor T cell responses, while a recent study in thyroid cancer patients highlights high PD-L1 expression and not IL-10 production by tumor PD-1 B cells as being responsible for T cell suppression and cancer survival (19, 30). An alternative suggested mechanism of action of B cell PD-1 that warrants further investigation is the possibility that PD-1 engages PD-L1 on the same cell in cis formation, resulting in reduced availability of PD-L1 for suppression of T cell activation during immunological synapse formation (31). We examined PD-L1 expression by RA patient–derived PD-1+ and PD-1– B cells and observed no differences in their capacity to express PD-L1. Although PD-1+ B cell IL-10 secretion was not assessed, these cells were more activated, evidenced by increased expression of CD86 and CD80, and produced high levels of several proinflammatory cytokines, including GM-CSF. Pathogenic GM-CSF–expressing B cells have recently been described in humans. GM-CSF expression is increased following in vitro BCR-mediated stimulation in the presence of surrogate T cell help, with multiple sclerosis patient–derived B cells showing significantly higher GM-CSF–secreting capacity than HC-derived B cells (32). The proinflammatory characteristics of RA patient–derived PD-1+ B cells were evident under normoxic, and more notably, under hypoxic conditions that more closely resembled the unique environment of the inflamed RA joint. RA patient PD-1+ B cells had higher activation of the AKT/mTOR/S6 pathway, leading to increased GLUT1 expression and glucose uptake under normoxic and hypoxic conditions compared with PD-1– counterparts. Deprivation of glucose led to loss of PD-1 expression, and STAT3 activation coupled with direct visualization of NAD in RA B cells revealed a potent glycolytic profile, delineating an important role of glycolysis for the maintenance of PD-1+ B cells. There is a paucity of data on the metabolic requirement of B cell activation and function, and the effect of glycolysis on B cell biology has only recently been explored. B cells, following stimulation, rapidly increased glycolysis in a GLUT1-dependent manner, paralleled with increased antibody-producing capacity (33). Humoral responses can be greatly influenced by changes in oxygen availability, with activated B cells becoming more glycolytic under hypoxic conditions (34). Importantly, B cells exposed to chronic B cell–activating factor and autoimmune-prone B cells maintain high glycolytic capacity, with deletion of Glut-1 leading to reduced B cell proliferation and impaired antibody production (33). The increased glycolytic capacity of PD-1 B cells could in addition to their activation/proliferation/cytokine secretion also affect the metabolites they are contributing to their microenvironment. Further studies analyzing the metabolite contribution and cytokine production of PD-1 B cells are required. While B cell infiltration of the joint correlates with disease activity and B cell depletion therapy leads to disease amelioration in autoantibody-positive and autoantibody-negative RA patients, B cells are a relatively small population of the immune infiltrate of the joint (5, 6). Further investigation of the role of B cells in synovitis and the particular role of RA joint PD-1 B cells is required. Additionally, a more expansive RA patient synovial biopsy sample size, inclusive of RA patients with high disease activity and paralleled with classification based on type of synovial infiltrate, would allow for the identification of relations between degree of PD-1 B cell infiltration, type of infiltrate, and synovitis. In conclusion, we provide evidence in support of early therapeutic intervention by inhibiting the role of CXCR3 in B cell migration to the synovial tissue and the potential for more specific B cell therapeutic targeting of pathogenic, glycolytic PD-1–expressing synovial B cells. We also highlight the importance of careful data extrapolation from normoxic to more physiologically relevant hypoxic conditions that closely resemble the unique environment of the inflamed joint. Methods Synovial tissue single-cell suspensions. Synovial biopsies (~15) were enzymatically and mechanically digested using the gentleMACS Tumor Dissociation Kit, human (Miltenyi Biotec), as per manufacturer’s instructions. Briefly, 15 synovial biopsies were placed in 4.7 mL of RPMI supplemented with 200 μL of enzyme H, 100 μL enzyme R, and 25 μL enzyme A in a gentleMACS C Tube followed by initial mechanical disruption of the tissue using program h_tumor_01 on a gentleMACS Dissociator. Samples were then incubated for a total of 1 hour at 37°C under continuous rotation using the MACSmix Tube Rotator with further applications of the gentleMACS Dissociator at the halfway point and at the end of the 1 hour incubation according to the manufacturer’s instructions. A single synovial cell suspension was generated by filtration through a 70 μm cell strainer. Matched PBMCs and SFMCs were also isolated using a density gradient preparation for direct comparison of B cell frequency in the circulation versus the inflamed synovium. Cell isolation and culture. B cells were isolated by magnetic bead cell sorting using negative (human B Cell Isolation Kit II, Miltenyi Biotec) or positive selection on the basis of CD19 expression (CD19 MicroBeads, human, Miltenyi Biotec) according to the manufacturer’s instructions. Purity of the isolated B cells was routinely checked by flow cytometric analysis for the detection of the B cell marker CD20 and was over 90%. Isolated B cells were then cultured in cRPMI (RPMI from Glutamax, Thermo Fisher Scientific), supplemented with 10% FBS (MilliporeSigma) and 1000 U/mL pen/strep (MilliporeSigma) in a 5% CO2 humidified incubator at 37°C under atmospheric O2 conditions or a humidified hypoxia chamber (5% CO2, 37°C) at 3% O2 as indicated. Cells were left unstimulated or were stimulated in vitro for 72 hours (at 1 × 106 cells/mL) with combinations of aCD40 (5 μg/mL, G28.5, InVivoMAb, Bio X Cell), F(ab′)2 aIgG + aIgM H and L chain cross-linking antibody (1 μg/mL, catalog 16-5099-85, Thermo Fisher Scientific), and CpG ODN2006 (0.2 μM, InvivoGen). Following incubation, supernatants were collected for cytokine analysis by ELISA, and cells were either analyzed by flow cytometric analysis or flow sorted (4-laser BD Aria flow sorter) on the basis of PD-1 expression for subsequent FLIM analysis. Flow cytometric analysis. Isolated and in vitro–cultured HC and RA patient–derived B cells, PBMCs, SFMCs, and synovial tissue single-cell suspensions were subjected to flow cytometric analysis. The generation of synovial tissue single-cell suspension was performed as previously described (18). Briefly, approximately 15 synovial biopsies per patient were enzymatically and mechanically digested using the gentleMACS dissociation kit (Miltenyi Biotec) as per manufacturer’s instructions. Cells were then passed through a 70 μm cell strainer before analysis. All extracellular targets were evaluated for loss of expression due to enzymatic digestion. Of all the targets tested, only CD27 was cleaved and expression was artificially lost. However, incubation of the synovial tissue single-cell suspensions for 6 hours postdigestion restored CD27 B cell expression (Supplemental Figure 1). Following the generation of single-cell suspensions, cells were washed in PBS and incubated with LIVE/DEAD fixable NIR (Thermo Fisher Scientific) viability reagent as per the manufacturer’s instructions. Cells were then incubated with TruStain FcX receptor blocking solution (BioLegend) to minimize nonspecific antibody binding. Next, cells were stained with antibody combinations targeting surface markers for 30 minutes at 4°C (Supplemental Table 1). Following incubation, cells were washed twice in FACS buffer (PBS with 2% FBS and 0.002% w/v sodium azide). If intracellular staining was required, cells were subsequently fixed and permeabilized using the intracellular Foxp3 staining kit (eBioscience, Thermo Fisher Scientific) as per the manufacturer’s instructions. Briefly, following incubation with the kit’s fix/perm buffer at 4°C for 30 minutes, cells were washed in perm buffer and incubated with antibody combinations for intracellular staining for 30 minutes at 4°C (Supplemental Table 1). Cells were then washed once with perm buffer and once with the FACS buffer before acquisition on a 4-laser LSRFortessa cytometer (BD). Glucose uptake and mitochondrial mass analysis. Sorted B cells were cultured in vitro under normoxic or hypoxic conditions and stimulated as described herein. For the last 30 minutes of culture, the cell culture medium was replaced with glucose-free RPMI (Thermo Fisher Scientific) supplemented with 1000 U/mL pen/strep (MilliporeSigma) and 50 μM of 2-NBDG (Invitrogen, Thermo Fisher Scientific). Prior to addition of the glucose-free cell culture medium, the media were left to equilibrate under the respective normoxic or hypoxic conditions of the cells. Following incubation, cells were washed in PBS, incubated with viability dye followed by Fc blocking step and extracellular staining as described herein, and then immediately acquired on a 4-laser Fortessa analyzer (BD). Relative mitochondrial mass was estimated based on incorporation of MitoTracker Green (Thermo Fisher Scientific). RA patient–derived B cells were isolated and stimulated under normoxic and hypoxic conditions as described herein. During the last 30 minutes of culture, cells were washed and resuspended in RPMI without FBS supplemented with 20 nM of MitoTracker Green. Cells were washed and stained for viability and expression of PD-1 before acquisition on a 4-laser Fortessa analyzer. Immunofluorescence. Synovial biopsies were fixed in 10% neutral-buffered formalin solution followed by paraffin embedding. Synovial tissue sections, 3 μm thick, were heated for 30 minutes at 60°C, deparaffinized in xylene, and rehydrated in alcohol and deionized water. Antigen retrieval was performed by heating sections in antigen retrieval solution (15 mL of 1 M sodium citrate and 15 mL of 1 M citric acid in deionized water, pH 6.0) in a pressure cooker. Slides were washed in PBS for 5 minutes. Nonspecific binding was blocked using 10% casein in PBS for 30 minutes. Primary antibodies aPD-1 (Abcam, clone: NAT105) and aCD19 (Thermo Fisher Scientific, clone: JF100-06) were incubated on sections for 2 hours at room temperature. An IgG1 control antibody (Dako) was used as a negative control. Slides were washed in PBS/Tween followed by 1-hour incubation at room temperature with secondary Cy2 (catalog 115-225-146) and Cy3 (catalog 111-165-144) AffiniPure secondary antibodies (Jackson ImmunoResearch). Slides were washed with PBS/Tween and PBS, before counterstaining of nuclei with DAPI (MilliporeSigma) and cover slide mounting with ProLong Gold Antifade (Thermo Fisher Scientific). Stained cells were visualized with a Leitz DM40 microscope (Leica Microsystems), and images were captured using the AxioCam system and AxioVision 3.0.6 software (Carl Zeiss Inc). ELISA. Tissue culture supernatants of sorted and in vitro–cultured B cells were analyzed by ELISA for the presence of IL-6 (DuoSet, R&D, Bio-Techne) or TNF-α (DuoSet, R&D Systems, Bio-Techne) according to the manufacturer’s instructions. PCR. RA patient and HC peripheral blood B cells were isolated and cultured under normoxic or hypoxic conditions as described herein. Total RNA was isolated using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. RNA quantification was performed on a NanoDrop spectrophotometer (Thermo Fisher Scientific). Samples with a 260/280 nm and 260/230 nm ratio of 1.8 or above were used for subsequent cDNA synthesis. Total RNA was reverse-transcribed to cDNA using the RT2 First Strand Kit (QIAGEN) as per manufacturer’s instructions. Genomic DNA elimination was performed as described previously (19). PCR was performed using the RT2 SYBR Green Mastermix (QIAGEN) as per the manufacturer’s instructions. PCR was performed on a LightCycler 480 System (Roche Diagnostics) with the following primers: MMP9 forward: ATTGGATCCAAAACTACTCGGAAGA, MMP9 reverse: GGGCAAAGGCGTCGTCAATC. Relative gene expression changes were determined by the 2−ΔΔCt method and normalized to the housekeeping gene RPLPO (primers: forward: GCGTCCTCGTGGAAGTGACATCG and reverse: TCAGGGATTGCCACGCAGGG) (all from Microsynth). Cell invasion assays. RA patient–derived B cells, isolated as described herein, were seeded at a density of 1.5 × 104 cells per well with aCD40 (5 μg/mL) in the migration chamber of a 24-well Corning Matrigel (8 μm membrane precoated with Matrigel, Thermo Fisher Scientific) with or without addition of the small molecular weight antagonist of CXCR3, AMG487 (200 μM, Tocris, Bio-Techne). Medium supplemented with or without 10% RA patient ex vivo synovial biopsy-conditioned media was added to the well. The cells were incubated for 24 hours. Noninvading cells remaining on the migration chamber were removed and counted by flow cytometric analysis using cell counting beads (CountBright absolute counting beads, Thermo Fisher Scientific) following LIVE/DEAD and CD20 staining. Cells that migrated from the insert to the corresponding well were similarly counted by flow cytometric analysis. Fluorescence lifetime imaging microscopy. Peripheral blood RA patient–derived B cells, isolated as described above, were stimulated in vitro for 72 hours with aCD40 (5 μg/mL), F(ab′)2 aIgG + aIgM H and L chain cross-linking antibody (1 μg/mL), and CpG ODN2006 (0.2 μM). Cells were cultured at 1 × 106 cells/mL. B cells were then flow sorted with a purity greater than 98% on the basis of PD-1 expression using a 4-laser Aria sorter (BD) and were immediately transferred to an 18-well 15-μ-Slide (Ibidi). FLIM utilizes endogenous fluorophores such as NAD in order to obtain an image. FLIM’s capacity to distinguish between bound and unbound NAD is based on the self-quenching process of NAD. In unbound NAD, the nicotinamide and adenine rings are in close proximity, and the fluorescence decay signal following excitation is approximately 0.4 ns. When in protein-bound form, NAD has a significantly longer fluorescence lifetime in the range of 2–4 ns due to stretching of the molecule, leading to longer distance between the nicotinamide and adenine rings and reduced self-quenching (20–22). FLIM was performed using an upright Olympus BX61W1 multiphoton microscopy system equipped with a Ti:Sapphire Laser (Chameleon Ultra, Coherent), a water-immersion objective (×25 Olympus 1.05 NA), and a temperature-controlled stage (37°C). NAD excitation was performed at a wavelength of 760 nm, and fluorescence emission was selected with a 455/90 nm bandpass filter. Fluorescence decay measurements were obtained using a PicoHarp 300 TCSPC system operating in the time-tagged mode coupled with a photomultiplier detector assembly hybrid detector (PicoQuanT GmbH) at 256 time bins per pixel. A 2-component fitting was used to differentiate between the free (τ1) and protein-bound (τ2) NAD(P)H: the average lifetime (τavg) of NAD(P)H for each pixel was calculated by a weighted average of both free and bound lifetime contributions: τavg = ([α1 × τ1] + [α2 × τ2])/(α1 + α2). RNA-Seq. Single-end 75-bp RNA-Seq at a read depth of 50,000,000 reads per sample was performed. Raw reads from FASTQ files were assessed for quality using FastQC. The reads were then pseudoaligned and transcript expression quantified (35) with kallisto. Transcripts were annotated with the Homo sapiens Ensembl version 86 build. The resulting transcript counts were used for differential analysis with edgeR after removal of transcripts with low counts. Testing for differential expression was performed using the generalized linear model options in edgeR (glmQFTest function in the Bioconductor edgeR package). Our RNA-Seq data have been deposited in the NCBI’s Gene Expression Omnibus database (accession number GSE154988). PCA plots of the samples based on their transcript profiles were plotted using the R stats and ggplot2 packages. Downstream pathway enrichment studies were performed using a variety of methods, including GSEA and gene set variation analysis as implemented in the corresponding R/Bioconductor package. Further information can be found in Supplemental Methods. Statistics. Statistical analysis was performed using Prism 7 (GraphPad) software. One-way or 2-way ANOVA with Tukey’s multiple-comparisons test and unpaired 2-tailed standard Student’s t test was used as indicated. Statistical significance was considered with P values of less than 0.05. Study approval. Peripheral blood, SF, and synovial tissue samples were collected from patients who were recruited from the Rheumatology Department, St. Vincent’s University Hospital; University College Dublin; and Tallaght University Hospital, Trinity College Dublin (Supplemental Table 2 and Supplemental Table 3). HC peripheral blood samples were obtained from buffy coats from St. James’s Hospital blood transfusion department and healthy volunteers recruited at Trinity Biomedical Sciences Institute and St. Vincent’s University Hospital. All subjects gave fully informed written consent approved by the institutional Ethics Committee, and research was performed in accordance with the Declaration of Helsinki. RA patient arthroscopies were performed under local anesthetic using Wolf 2.7 mm needle arthroscopy or ultrasound-guided biopsy as previously described (17). Author contributions AF designed and performed experiments, analyzed data, and wrote the manuscript. NN performed FLIM assay and analyzed data. VM performed experiments. KM recruited patients and obtained patient samples. BM performed flow cytometric cell sorting. MGM analyzed data and wrote the manuscript. CL recruited patients and obtained patient samples. RHM recruited patients and obtained patient samples. NR analyzed data. VK performed RNA-Seq data analysis and wrote the manuscript. SN analyzed data and wrote the manuscript. DJV recruited patients and obtained patient samples, analyzed data, and wrote the manuscript. UF designed experiments, analyzed data, and wrote the manuscript. Supplemental material View supplemental data Acknowledgments Graphical abstract created with BioRender. This work was supported by the Health Research Board (ILP-POR-2017-047) and Arthritis Ireland. Footnotes Conflict of interest: NR, VK, and SN are current or former employees of Janssen Research & Development, Johnson & Johnson. Copyright: © 2020, Floudas et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License. Reference information: JCI Insight. 2020;5(21):e139032.https://doi.org/10.1172/jci.insight.139032. References Aletaha D, Blüml S. Therapeutic implications of autoantibodies in rheumatoid arthritis. RMD Open. 2016;2(1):e000009. View this article via: PubMed CrossRef Google Scholar Guo Y, et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One. 2018;13(2):e0192704. View this article via: PubMed CrossRef Google Scholar Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123(11):1904–1911. View this article via: PubMed CrossRef Google Scholar Cohen MD, Keystone E. Rituximab for rheumatoid arthritis. Rheumatol Ther. 2015;2(2):99–111. View this article via: PubMed CrossRef Google Scholar Mease PJ, et al. Efficacy and safety of retreatment in patients with rheumatoid arthritis with previous inadequate response to tumor necrosis factor inhibitors: results from the SUNRISE trial. J Rheumatol. 2010;37(5):917–927. View this article via: PubMed CrossRef Google Scholar Lewis MJ, et al. Molecular portraits of early rheumatoid arthritis identify clinical and treatment response phenotypes. Cell Rep. 2019;28(9):2455–2470.e5. View this article via: PubMed CrossRef Google Scholar Humby F, et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann Rheum Dis. 2019;78(6):761–772. View this article via: PubMed CrossRef Google Scholar Váncsa A, et al. Longterm effects of rituximab on B cell counts and autoantibody production in rheumatoid arthritis: use of high-sensitivity flow cytometry for more sensitive assessment of B cell depletion. J Rheumatol. 2013;40(5):565–571. View this article via: PubMed CrossRef Google Scholar Haraoui B, Cividino A, Stewart J, Guérette B, Keystone EC. Safety and effectiveness of adalimumab in a clinical setting that reflects Canadian standard of care for the treatment of rheumatoid arthritis (RA): results from the CanACT study. BMC Musculoskelet Disord. 2011;12:261. View this article via: PubMed Google Scholar Gerlag DM, et al. Effects of B-cell directed therapy on the preclinical stage of rheumatoid arthritis: the PRAIRI study. Ann Rheum Dis. 2019;78(2):179–185. View this article via: PubMed CrossRef Google Scholar Cohen SB, et al. Continued inhibition of structural damage over 2 years in patients with rheumatoid arthritis treated with rituximab in combination with methotrexate. Ann Rheum Dis. 2010;69(6):1158–1161. View this article via: PubMed CrossRef Google Scholar Palanichamy A, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol. 2014;193(2):580–586. View this article via: PubMed CrossRef Google Scholar Schuh E, et al. Features of human CD3+CD20+ T cells. J Immunol. 2016;197(4):1111–1117. View this article via: PubMed CrossRef Google Scholar Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107(10):4658–4663. View this article via: PubMed CrossRef Google Scholar Anolik JH, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56(9):3044–3056. View this article via: PubMed CrossRef Google Scholar Palanichamy A, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182(10):5982–5993. View this article via: PubMed CrossRef Google Scholar Ng CT, et al. Synovial tissue hypoxia and inflammation in vivo. An

|
Scooped by
Gilbert C FAURE
July 23, 2020 7:09 AM
|
Osteoarthritis (OA) is a complex musculoskeletal disease and a leading cause of pain and disability worldwide. Hip and knee OA alone are major contributors to global disability, having notable effects on individual well-being, increasing the reliance of individuals on health-care services and contributing to a rise in the socioeconomic burden. Consistent, coordinated and tailored approaches are important for providing appropriate care to all people with OA, but despite the scale of the challenge many individuals are still not offered the safe, best-evidence treatments recommended for OA care. This Review discusses the core priority treatments for OA, including exercise and physical activity, weight-loss, education and support for self-management. Additional physical or psychological evidence-based adjunctive therapies and combined therapies that can be used to tailor individual programmes are also discussed. These options include cognitive behavioural therapy, heat therapy, walking aids and splints, manual therapies and transcutaneous electrical nerve stimulation. International examples of OA treatment options, models of care and resources available are also given. Many challenges still need to be addressed to advance the uptake of these conditions, including further discussion around the risks and costs involved with all treatments. Various core and adjunctive therapies are available for osteoarthritis (OA) that can have beneficial effects on the well-being of the individual; however, challenges remain in implementing best-evidence, high-value care.

|
Scooped by
Gilbert C FAURE
May 20, 2020 5:30 AM
|
Arthritis Rheumatol. Author manuscript; available in PMC 2016 Dec 28. Published in final edited form as: doi: 10.1002/art.39458 PMCID: PMC5195265 NIHMSID: NIHMS835582 PMID: 26473401 Enthesitis New Insights Into Pathogenesis, Diagnostic Modalities, and Treatment The publisher's final edited version of this article is available free at Arthritis Rheumatol See other articles in PMC that cite the published article. Introduction Enthesitis is a central feature of spondyloarthritis (SpA). Although enthesitis has traditionally been considered to be a focal insertional disorder, advanced imaging and pathologic findings suggest that enthesitis is a diffuse process with effects on adjacent bone and soft tissue. As a result of repeated biomechanical stress, it appears that microdamage at the enthesis triggers an inflammatory response in the synovium, leading to synovitis. Along with mechanical stress, exogenous bacteria may play a role in activating the immune response, especially in genetically predisposed individuals whose major histocompatibility locus encodes the class I molecule HLA–B27. Recent studies in animal models suggest that autoimmunity against versican and fibrocartilage proteins, and bone morphogenetic protein (BMP) signaling play roles in enthesitis development. Finally, interleukin-23 (IL-23) has been implicated in enthesitis with inflammatory effects mediated through IL-17 and tumor necrosis factor (TNF), and new bone formation driven by IL-22. Although prior therapeutic choices were limited to nonsteroidal antiinflammatory drugs (NSAIDs) and activity modification, in recent years TNF inhibitors have proven to be useful. Further research on the effects of IL-22 and IL-23 blockade is needed to understand the effects on the treated patient. While enthesitis is underdiagnosed by physical examination alone, the use of ultrasound has proven to be highly sensitive for the detection of enthesitis, with utility in monitoring response to therapy, and will be an invaluable tool for assessing the efficacy of newer treatments. This review summarizes the substantial progress that has been made in addressing the pathophysiology, molecular mechanisms, genetic associations, clinical features, diagnostic modalities, and treatment of enthesitis. Definitions and evolution of the enthesis concept Historic definition Although the adjective “enthetic” derives from the ancient Greek word “enthetikos,” meaning “introduced into the body from without,” in the nineteenth century the adjective was increasingly used to refer to diseases that were “implanted into the body from external sources” (1). It was not until the twentieth century that the term “enthesis” was used as it is today, referring to focal insertional abnormalities at sites of bony attachments to tendons, ligaments, fascia, muscles, or joint capsules (2,3). The first suggestion that the enthesis is centrally affected in SpA was made by Ball in 1971 and was substantiated after a review of pathologic tissues from both patients with rheumatoid arthritis (RA) and patients with ankylosing spondylitis (AS), where he noted the presence of a unique inflammatory enthesopathy that could help to distinguish SpA from RA (2). Broadening the definition of enthesis with the concept of the “enthesis organ” Magnetic resonance imaging (MRI) and ultrasound findings have suggested that enthesopathy encompasses pathologic changes extending to the adjacent bone and soft tissues (4). Likewise, it has been argued that this entity should be considered an “enthesis organ” encompassing not only the enthesis itself, but also the fibrocartilage, bursa, fat pad, adjacent trabecular bone networks, and deeper fascia (5) (Figure 1). Representing areas where hard and soft tissues meet, entheses are sites of concentrated stress with effects not only on the bony attachment interface and the enthesis itself, but also on these neighboring tissues (4–7). Entheses and mechanical stress The concept of an enthesis organ was extended to that of a synovioentheseal complex (8,9), which refers to the relationship between the proinflammatory synovium and the avascular enthesis. In contrast to other skeletal locations, the enthesis is a site of repetitive biomechanical forces. High biomechanical stress at the enthesis triggers an inflammatory cascade with cytokine production by infiltrating monocytes and lymphocytes in the adjacent synovial tissue, resulting in an articular inflammatory response, and clinically leading to synovitis adjacent to attachment sites (8,9). Support for this theory of a dynamic response to biomechanical stress at the enthesis originates from animal models. In one experiment, botulinum toxin A injection delayed fibrocartilage development, suggesting that enthesis development is sensitive to mechanical environmental factors (10). In a mouse model that overexpresses TNF, enthesitis was reduced when the hind legs of the mice were made non–weight-bearing through tail suspension (11). Those authors proposed that triggering of mechanoreceptors via the MAPK pathway stimulates the production of inflammatory mediators. As a result of biomechanical stress, adjacent bone reacts with formation of surface spurs or enthesophytes, observed both radiographically and on histologic examination (12). In early disease, there is destruction of superficial fibrocartilage, with vascular invasion and inflammatory cell infiltration, predominantly with macrophages (13). This leads to another important microanatomical feature, which is the presence of blood vessels at sites where synovium, subchondral bone, and bone marrow are close to each other. In early experiments using labeled phosphorus, Ball identified capillary-like vessels that pass through the enthesis to the marrow (2). Later studies described the presence of vascular channels penetrating cortical bone in the knees of mice adjacent to the cruciate ligaments with associated subclinical changes, including subchondral bone damage and microcyst formation. In the rat adjuvant-induced arthritis model, vascular channels provided a site for inflammatory tissue entry and osteoclast activation (14). Whether enthesitis is a primary central lesion or a secondary process remains a matter of debate. Studies that have implicated enthesitis as the primary process include studies of TNF-transgenic mice, in which the earliest lesion appears to be in the enthesis (11). However, this may be model specific, and a number of reports have challenged the idea of enthesitis as the primary inflammatory lesion (15,16). In one study examining different stages of spontaneous tail spondylitis and peripheral arthritis in HLA–B27/hβ2m–transgenic mice, histologic samples displayed destructive synovitis with neutrophils and multinucleated giant cells rather than by enthesitis or osteitis (16). Among human studies examining biopsy specimens and MRIs of sacroiliac joints, synovitis and subchondral bone marrow changes were more prominent features while enthesitis was not (17,18). In a subsequent study, in patients with early untreated knee or ankle arthritis, analyses revealed a higher synovitis score by MRI in SpA than in RA, whereas there were no differences in the prevalence of enthesitis as assessed by perientheseal focal tissue, entheseal enhancement, and bone marrow edema (15). However, in light of substantial data in animal models highlighting 3 stages of tendon response to injury that have been defined by distinct pathologic changes, determining the initiating event in the enthesis may be confounded by the timing of the analysis (19–21). Contributing cellular and molecular mechanisms Genetic susceptibility It has long been known that AS susceptibility is largely genetically determined. The strongest genetic association is with the major histocompatibility complex (MHC)–encoded class I molecule, HLA–B27, and it is postulated that HLA–B27 contributes to ~40% of the overall risk for SpA (22). Protein misfolding of nascent HLA–B27 in the endoplasmic reticulum has been hypothesized to trigger an unfolded protein response with aberrant recognition by natural killer cell receptors (23). The HLA–B27–induced unfolded protein response in macrophages has been demonstrated in HLA–B27–transgenic rats and is associated with an increase in IL-23 production by these cells (24). Although HLA–B27 remains the dominant risk factor for susceptibility to the AS phenotype, other important influences of the MHC have been observed (25). More recently, Haroon et al (26) found a positive association of B*27:05:02 with enthesitis, dactylitis, and symmetric sacroiliitis in a cohort of psoriatic arthritis (PsA) patients, whereas B*44 haplotypes were associated with a decreased frequency of enthesitis, dactylitis, and joint fusion. Finally, investigators have recently focused on genes outside of the MHC region, such as ERAP1 and ERAP2, which code for aminopeptidases that are involved in MHC class I presentation (25,27). Although additional HLA class I and class II alleles have also been implicated, the scale and scope of gene identification to date have not yet matched the putative total genetic risk for SpA. Microbial factors Microbial infection with virulent organisms remote from affected joints, as well as gastrointestinal dysbiosis without a directly invading pathogen, are known features of certain phenotypes of SpA, and it has long been appreciated that microbial factors can lead to immune activation (28). Clinically, reactive arthritis (ReA) is known to follow infections with Chlamydia, Campylobacter, Shigella, or Yersinia. AS patients consistently have been found to have subclinical gut inflammation and increased gastrointestinal permeability (29,30). In animal models, HLA–B27–transgenic rats raised in germ-free environments do not develop intestinal inflammatory or peripheral joint disease, yet the disease recurs if rats are reconstituted with Bacteroides, supporting the role of gut flora in the development of joint inflammation (31). In a more recent study, colonoscopic biopsies of the terminal ileum of AS patients showed a discrete microbial signature as revealed by sequencing and quantitative polymerase chain reaction analysis of the 16S ribosomal RNA (16S rRNA) gene, exhibiting higher levels of 5 families of bacteria as compared to healthy controls (32). In that study there was no significant difference in the 16S rRNA copy number between patients with AS and controls, indicating that the observed differences were not due to bacterial overgrowth. It has been postulated that the combination of bacterial adjuvants and mechanical factors act synergistically to activate the immune response, particularly in genetically predisposed individuals (5). Fibrocartilage and versican autoimmunity A number of studies have indicated that autoimmunity against fibrocartilage proteins, including aggrecan, may underlie enthesitis and spondylitis (33). A model of SpA induced by immunizing BALB/c mice with the G1 globular domain of versican, leading to spondylitis and enthesitis, suggests that versican autoimmunity may also play a role in enthesitis (34). The inflammatory lesions are characterized by mononuclear cell infiltration at the entheseal insertions to the vertebrae, as is seen with AS, and are associated with angiogenesis which then progresses to cause destructive discitis (35). Role of bone morphogens In the DBA/1 mouse model, where mice develop spontaneously occurring arthritis that culminates in bone formation and joint ankylosis, male mice in crowded conditions developed arthritis in the hind paws that was entheseal, but not synovially based, with new bone formation driven by BMP-7 signaling (36). In that experiment, the incidence of arthritis was increased in mice that were caged together in crowded conditions, yet decreased when the mice were placed in larger cages (37). Thus, in addition to a genetic predisposition for enthesitis, this observation points to the role of environmental factors in the development of arthritis. Finally, immunohistochemical studies in SpA show increased synovial expression of BMP-2 and BMP-6, which is up-regulated by proinflammatory cytokines such as IL-1 and TNF, suggesting that synovial molecules contribute to chronic arthritis and joint ankylosis (36,38). Role of proinflammatory cytokines The role of IL-23 has been addressed as a major driver of cascades that lead to inflammation and bone remodeling in SpA. Alterations in AS susceptibility are related to the existence of single-nucleotide polymorphisms in the IL-23 receptor as demonstrated in genome-wide association studies, and serum levels of the IL-12/23 p40 subunit have been shown to be significantly higher in patients with PsA compared with controls (39,40). IL-23 is produced in the gut, suggesting that the intestinal mucosa is a key site of IL-23 production in SpA. Additionally, Chlamydia trachomatis also leads to induction of IL-23 via CHOP10. Taken together, these findings indicate that IL-23 is a pivotal cytokine and potentially central to the pathogenesis of SpA (41). Increased IL-17 expression by innate immune cells such as mast cells and neutrophils in SpA has been shown to target the facet joints and synovial tissue (42,43). In a subsequent set of investigations, Sherlock et al found that IL-23 could induce SpA by acting on an isolated population of CD3+CD4−CD8− entheseal resident lymphocytes, leading to increased expression of TNF and IL-6 in the enthesis. When IL-23 was overexpressed, mice developed enthesitis with inflammation, which spread into the adjacent synovium (41). Enthesitis was associated with new bone erosion. IL-23 promoted inflammation through IL-17 and TNF, whereas new bone formation was associated with overproduction of IL-22 (41,44) (Figure 2). Additional support for the role of IL-23 comes from the SKG mouse model, in which curdlan (β-1,3-glucan) injections induce enthesitis and dactylitis. Arthritis and spondylitis were IL-23 dependent and were transferable to SCID mouse recipients with CD4+ T cells (45). In this model, disease severity was dependent on the external microbial environment and the host immunogenetic background. More recent work illustrates the differential impact of microbiota on specific pathologic features of SpA; ileitis development, ileal IL-23 expression, and lymph node IL-17A production were microbiota dependent, but arthritis was not (46). In curdlan-treated SKG mice, enthesitis was specifically dependent on IL-17A and IL-22 (47). The role of up-regulation of the IL-23/Th17 pathway in promoting joint inflammation and bone turnover is further supported by recent murine studies, with inhibition of the PsA phenotype after neutralization of IL-17A (48,49). Clinical enthesitis in SpA SpA is by definition a heterogeneous group of clinical entities long recognized as having unique phenotypes that include AS, ReA, PsA, enteropathic arthritis, and what has traditionally been referred to as undifferentiated arthritis. However, with advances in imaging and careful long-term followup observations, it appears that these diseases share common features, including subclinical spinal and peripheral joint inflammation, along with associations with microbes and gene identifications. In attempting to develop a model for an underlying unifying anatomical basis for SpA, an “enthesitis-based model” has been proposed as the basis for the osteitis, periostitis, and new bone formation that are seen in SpA (5). The association between enthesitis and adjacent osteitis has been further supported by imaging and cadaver studies, primarily in patients with PsA (50–52). Regional sites Patients with SpA have a remarkable propensity for inflammation at certain enthesis sites that are ubiquitous and numerous. Clinically, peripheral enthesitis is observed not only in all forms of SpA, but particularly frequently in juvenile-onset SpA. A number of patients with juvenile SpA are classified as having enthesitis-related arthritis (ERA), a heterogeneous subtype that includes some patients who predominantly have enthesitis, enthesitis and arthritis, or juvenile AS. Compared to other subtypes of juvenile idiopathic arthritis, ERA is associated with worse function, worse quality of life, and increased pain (53,54). Enthesitis can be seen in 33–58% of patients with ReA and may be the only clinical manifestation in some whose disease has been triggered by an enteric infection (55). In SpA, the entheses of the lower extremities are more frequently involved than those of the upper limbs, and the heel is the most frequent site (55). In addition to the Achilles and plantar fascia insertions, identified sites of enthesitis include muscle attachments to the greater and lesser trochanters, the insertion of the quadriceps tendon at the upper patellar pole, the insertions of the patellar ligament at the lower patellar pole and the tibial tubercle, acromial and clavicular insertions of the deltoid muscle, and the insertions of the flexor and extensor tendons at the phalanges (55–57). It is unknown why there is a predilection for the entheses at the lower parts of the lower limbs, although it has been hypothesized that this may be due to the length, anatomy, and higher mechanical load at these sites. Given the presumed role of repetitive biomechanical forces discussed above, it is not surprising that in patients with longstanding AS, those with occupational activities that required more bending, twisting, and stretching had more functional limitations and radiographic damage than those whose jobs required little or no dynamic flexibility (58). A recently published computer-based method that fully quantified syndesmophyte heights and volumes on computed tomography scans has revealed that syndesmophytes grow at different rates over time in AS patients, suggesting that mechanical factors local to the disc space may influence syndesmophyte formation (59). Clearly, there are sites that are not associated with SpA despite being sites of significant biomechanical stress, and perhaps it is the compressive and shear force nature of the stress as well as the putative role of antigen expression adjacent to the enthesis that may underlie this apparent discrepancy (5). Additionally, it cannot be discounted that the increased detection of enthesitis at the lower limbs is explained by the accessibility of these sites to ultrasound. Diagnostic criteria and outcome measures Enthesitis is often underdiagnosed in the clinic; clinical assessment and quantification of peripheral enthesitis in daily practice lacks sensitivity and specificity (56,60,61). Although both the Amor criteria (62) and the European Spondylarthropathy Study Group criteria (63) for SpA include peripheral enthesitis, there are limitations to these criteria with regard to the exact quantification of enthesitis. Two clinical methods have been designed and often implemented for evaluating enthesopathy in AS: Mander’s Entheseal Index and the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) (64,65). Both rely on pain elicited by local pressure of entheseal points. The intraarticular and deep location of entheseal insertions, however, makes quantification of enthesitis by physical examination alone difficult, and not surprisingly, these scoring systems have only moderate sensitivity and specificity for predicting positive sonogram results, depending on the entheseal site (66). Imaging of the enthesis Because of the clinical limitations described above and the poor sensitivity of markers of inflammation, it is necessary to rely on typical abnormalities seen on various imaging techniques to diagnose SpA. Plain radiographs are limited by their inability to show inflammation or soft tissue changes, although late chronic bony changes such as enthesophyte formation or occasional erosions can be seen at the attachment of the Achilles tendon or plantar aponeurosis. More sensitive methods such as ultrasound and MRI, which are useful in their ability to detect both inflammatory and chronic changes in enthesitis at both early and late stages, can be used. MRI MRI has changed the way we approach both the diagnosis and classification of SpA; it is particularly useful in detecting spinal disease in early AS when conventional radiographs are still normal (67). The use of fat-suppressed, fat-saturated, and water-sensitive MRI sequences has demonstrated that the extracapsular inflammation of joints quite often represents enthesitis with variable degrees of soft tissue and bone marrow edema (68,69) (Figures 3–5). The typical appearance of enthesitis on MRI includes soft tissue inflammatory changes outside the joint capsule and perientheseal bone marrow edema (70). Recent studies have examined the utility of whole-body MRI, which has shown promise in the detection of subclinical axial and peripheral enthesitis (71). Of course, MRI has limitations; structures that make up entheses have a low signal on conventional MRI, with low water accumulation in the areas where fibroblasts are tightly cross-linked. MRI is further limited by its cost and availability, and therefore ultrasound remains the preferred modality for the detection of enthesitis both in the clinical setting as well as in research. Ultrasound Ultrasound has indeed proven to be a highly useful and sensitive tool in the evaluation of enthesitis and improves the ability of the clinical examination to detect enthesopathy. In one study of 92 patients with PsA, ultrasound was useful in detecting subclinical entheseal involvement, independent of clinical examination and symptoms (72). In another study of 600 lower limb entheses, at least 1 ultrasound sign of enthesopathy was detected in 60% of clinically asymptomatic cases of enthesitis, thus demonstrating a higher sensitivity than physical examination (73). Ultrasound may be most useful in the early diagnosis of SpA, and likewise, entheseal abnormalities can be detected prior to overt clinical disease. Nevertheless, in an older cross-sectional single-center study of 51 SpA patients and 24 controls, neither MRI nor power Doppler ultrasound (PDUS) discriminated between SpA and controls (74). In a prospective single-center cohort study of 118 patients with symptoms suggestive of SpA conducted by D’Agostino and colleagues (57), vascularization at cortical bone detected by PDUS of at least one enthesis provided good predictive value for diagnosing SpA with a sensitivity of 76.5% and a specificity of 81.3%. Indeed, PDUS is a sensitive and reliable technique used to detect increased blood flow in the enthesis revealing neovascularity and subclinical active inflammation (56,75) (Figure 6). Recent studies have indicated that ultrasound may accurately predict which patients will go on to develop SpA (51,57,75). In one investigation, ultrasound examination of Achilles erosions correlated with objective activity-based measurements of SpA outcomes, and was sensitive to change (76). In the study by D’Agostino and colleagues described above, vascularized enthesis as detected by PDUS combined with Amor’s criteria proved to be the only independent contributors to a diagnosis of SpA (57). Finally, ultrasound may be used to monitor response to therapeutic interventions. A few studies have illustrated improvement in enthesitis shown on ultrasound after the use of TNF antagonists (77,78). In one investigation of 327 patients with active SpA who were treated with anti-TNF therapy for 6 months, cumulative entheseal morphologic abnormalities, intraenthesis and perienthesis, and bursitis were all significantly decreased on PDUS after 6 months of treatment (77). In another study, D’Agostino et al monitored regression of enthesitis using PDUS after treatment with infliximab (78), providing confirmatory evidence for the utility of ultrasound in a clinical research setting. Treatment of enthesitis Historically, treatment of clinical enthesitis had been limited to NSAIDs. Continuous use of NSAIDs not only controls symptoms of disease, but may also slow progression of bony changes in AS (79,80). Therefore, Assessment of SpondyloArthritis international Society/European League Against Rheumatism guidelines place optimal NSAID therapy as a cornerstone of the management plan for AS (81). Treatment with TNF inhibitors is indicated in patients that do not respond to NSAID therapy. TNF inhibition with adalimumab, etanercept, infliximab, and golimumab has been shown to be efficacious in the treatment of enthesitis (82–87). Olivieri et al (88) have reported that adalimumab and etanercept are effective treatments of MRI-documented refractory heel enthesitis, with progressive improvement of bone edema in a 6-month period (88). Agents that block IL-23 have the potential to inhibit both inflammation and altered bone remodeling, although further analysis of the effect of IL-22 and IL-23 blockade on bone pathologies in animal models and patients with PsA are needed to address this important therapeutic issue (89). Entheseal inflammation in a passive-transfer model of collagen antibody-induced arthritis was reduced by an antibody to the p19 subunit of IL-23, which was also associated with the down-regulation of several inflammatory mediators, such as IL-6 and IL-1β, and genes such as Rankl, Ctsk, and matrix metalloproteinases known to be involved in bone erosion (41). Both ustekinumab, a monoclonal antibody directed against the common p40 subunit of IL-12 and IL-23, and secukinumab, a human anti–IL-17A monoclonal antibody, have already demonstrated promise in PsA, with significant improvements in enthesitis (90,91). Apremilast, an oral inhibitor of phosphodiesterase 4, which increases cAMP and thus modulates multiple proinflammatory mediators, has demonstrated efficacy in PsA, with significant improvements in the severity of both enthesitis and dactylitis evidenced by reductions in MASES over a 52-week period (92). Finally, bisphosphonates may also have a role in peripheral enthesitis felt to be refractory to NSAID therapy. In a 6-month randomized controlled comparison of intravenous pamidronate treatment of NSAID-refractory AS, patients treated with pamidronate showed symptomatic improvement with significant reductions in Bath Ankylosing Spondylitis Functional Index and Bath Ankylosing Spondylitis Disease Activity Index measurements together with regression of periarticular osteitis documented by MRI with gadolinium (93). Treatment of patients with SpA enthesitis with currently available agents has not had universal success. In placebo-controlled trials of methotrexate and leflunomide in PsA, enthesitis measures were not assessed (94,95). In a randomized controlled trial, sulfasalazine was not effective for enthesitis (96). Other agents that have not demonstrated clinical efficacy in AS include tocilizumab, and lymphocyte-targeted therapies such as abatacept (97,98). Rituximab only showed modest therapeutic efficacy in SpA (99,100). Conclusions In summary, investigations and clinical observations uniformly point out with increasing clarity that the enthesis is much more than a simple attachment site. A number of studies have shown that it functions as a unit comprising adjacent tissues, including bone and fibro-cartilage linked to synovium, and serves as a way of dissipating stress over a wide area. Inflammation at the enthesis manifests in the adjacent synovium presumably via immunity to common antigens or via release of proinflammatory cytokines at the enthesis. Although work by Benjamin and McGonagle (9) suggests that the enthesis is the primary SpA lesion, the precise role of the enthesis in early stages of disease, especially regarding issues of cause or effect, remains an area of continued debate and discovery. Improved imaging modalities may in the future be able to detect enthesitis at different stages of disease. However, this will require a clinically diverse and large sample size to help address this question. Inflammation at the enthesis is likely modulated by multiple factors. A more complete role for genetic predisposition will require additional advances in gene sequencing and discovery. Repeated biomechanical stress with the resultant inflammatory response regulated by IL-17, IL-22, and IL-23 now provide clues as to why certain areas of the body are affected, and perhaps why others are not. The spine itself (the clinical hallmark of the disease) remains inaccessible to traditional enthesitis-focused research methodologies thus far. However, newer imaging techniques are on the horizon. Further examination into the role of the inflammatory mediators, including IL-17, IL-22, and IL-23 as well as potentially others, in driving enthesitis and bone formation will be important to direct our attention toward future therapeutic targeted pathways in patients with SpA. Acknowledgments Supported in part by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant P01-AR-052915 and National Center for Advancing Translational Sciences grant UL1-TR-000124). The authors wish to thank Joseph Robinson, MD (Cedars-Sinai Medical Center Department of Radiology) for assistance with MRI acquisition and interpretation. Footnotes AUTHOR CONTRIBUTIONS All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. References 1. Lampman JH. Origin of enthesopathy. J Rheumatol. 1985;12:1030–1. [PubMed] [Google Scholar] 2. Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30:213–23. [PMC free article] [PubMed] [Google Scholar] 3. Francois RJ, Braun J, Khan MA. Entheses and enthesitis: a histopathologic review and relevance to spondyloarthritides. Curr Opin Rheumatol. 2001;13:255–64. [PubMed] [Google Scholar] 4. Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50:3306–13. [PubMed] [Google Scholar] 5. McGonagle D, Stockwin L, Isaacs J, Emery P. An enthesitis based model for the pathogenesis of spondyloarthropathy: additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol. 2001;28:2155–9. [PubMed] [Google Scholar] 6. Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–26. [PMC free article] [PubMed] [Google Scholar] 7. McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol. 2009;23(Suppl 1):9–13. [PubMed] [Google Scholar] 8. McGonagle D, Lories RJ, Tan AL, Benjamin M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007;56:2482–91. [PubMed] [Google Scholar] 9. Benjamin M, McGonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv Exp Med Biol. 2009;649:57–70. [PubMed] [Google Scholar] 10. Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development [published erratum appears in J Orthop Res 2009;27:141] J Orthop Res. 2007;25:1154–63. [PubMed] [Google Scholar] 11. Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. 2014;73:437–45. [PubMed] [Google Scholar] 12. Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the Achilles tendon. Arthritis Rheum. 2000;43:576–83. [PubMed] [Google Scholar] 13. McGonagle D, Marzo-Ortega H, O’Connor P, Gibbon W, Hawkey P, Henshaw K, et al. Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann Rheum Dis. 2002;61:534–7. [PMC free article] [PubMed] [Google Scholar] 14. Binks D, Matzelle M, Bergin D, Hodgson RJ, Tan AL, Gravallese EM, et al. The frequency of bone marrow oedema adjacent to the cruciate ligament peri-entheseal vascular channels in inflammatory and degenerative arthritis [abstract] Arthritis Rheum. 2013;65(Suppl):S25–6. [Google Scholar] 15. Paramarta JE, van der Leij C, Gofita I, Yeremenko N, van de Sande MG, de Hair MJ, et al. Peripheral joint inflammation in early onset spondyloarthritis is not specifically related to enthesitis. Ann Rheum Dis. 2014;73:735–40. [PubMed] [Google Scholar] 16. Van Duivenvoorde LM, Dorris ML, Satumtira N, van Tok MN, Redlich K, Tak PP, et al. Relationship between inflammation, bone destruction, and osteoproliferation in the HLA–B27/human β2-microglobulin–transgenic rat model of spondylarthritis. Arthritis Rheum. 2012;64:3210–9. [PMC free article] [PubMed] [Google Scholar] 17. Francois RJ, Gardner DL, Degrave EJ, Bywaters EG. Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis: systematic study of specimens from patients and control subjects. Arthritis Rheum. 2000;43:2011–24. [PubMed] [Google Scholar] 18. Muche B, Bollow M, Francois RJ, Sieper J, Hamm B, Braun J. Anatomic structures involved in early- and late-stage sacroiliitis in spondylarthritis: a detailed analysis by contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003;48:1374–84. [PubMed] [Google Scholar] 19. Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–16. [PubMed] [Google Scholar] 20. Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets: a mini-review. Int Orthop. 2007;31:783–9. [PMC free article] [PubMed] [Google Scholar] 21. Apostolakos J, Durant TJ, Dwyer CR, Russell RP, Weinreb JH, Alaee F, et al. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4:333–42. [PMC free article] [PubMed] [Google Scholar] 22. Reveille JD. The genetic basis of spondyloarthritis. Ann Rheum Dis. 2011;70(Suppl 1):i44–50. [PubMed] [Google Scholar] 23. Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57:44–51. [PMC free article] [PubMed] [Google Scholar] 24. DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA–B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–43. [PMC free article] [PubMed] [Google Scholar] 25. Cortes A, Pulit SL, Leo PJ, Pointon JJ, Robinson PC, Weisman MH, et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat Commun. 2015;6:7146. [PMC free article] [PubMed] [Google Scholar] 26. Haroon M, Winchester R, Giles JT, Heffernan E, FitzGerald O. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann Rheum Dis. 2014 E-pub ahead of print. [PubMed] [Google Scholar] 27. Breban M, Costantino F, Andre C, Chiocchia G, Garchon HJ. Revisiting MHC genes in spondyloarthritis. Current Rheumatology Reports. 2015;17:516. [PubMed] [Google Scholar] 28. Hacker G, Redecke V, Hacker H. Activation of the immune system by bacterial CpG-DNA. Immunology. 2002;105:245–51. [PMC free article] [PubMed] [Google Scholar] 29. Jacques P, Van Praet L, Carron P, Van den Bosch F, Elewaut D. Pathophysiology and role of the gastrointestinal system in spondyloarthritides. Rheum Dis Clin North Am. 2012;38:569–82. [PubMed] [Google Scholar] 30. Matzkies FG, Targan SR, Berel D, Landers CJ, Reveille JD, McGovern DP, et al. Markers of intestinal inflammation in patients with ankylosing spondylitis: a pilot study. Arthritis Res Ther. 2012;14:R261. [PMC free article] [PubMed] [Google Scholar] 31. Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. [PMC free article] [PubMed] [Google Scholar] 32. Costello ME, Ciccia F, Willner D, Warrington N, Robinson PC, Gardiner B, et al. Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 2015;67:686–91. [PubMed] [Google Scholar] 33. Guerassimov A, Zhang Y, Banerjee S, Cartman A, Webber C, Esdaile J, et al. Autoimmunity to cartilage link protein in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 1998;25:1480–4. [PubMed] [Google Scholar] 34. Shi SL, Ciurli C, Cartman A, Pidoux I, Poole AR, Zhang Y. Experimental immunity to the G1 domain of the proteoglycan versican induces spondylitis and sacroiliitis, of a kind seen in human spondylarthropathies. Arthritis Rheum. 2003;48:2903–15. [PubMed] [Google Scholar] 35. Zhang YP, Guerassimov A, Leroux JY, Cartman A, Webber C, Lalic R, et al. Arthritis induced by proteoglycan aggrecan G1 domain in BALB/c mice. Evidence for T cell involvement and the immunosuppressive influence of keratan sulfate on recognition of T and B cell epitopes. J Clin Invest. 1998;101:1678–86. [PMC free article] [PubMed] [Google Scholar] 36. Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–9. [PMC free article] [PubMed] [Google Scholar] 37. Braem K, Carter S, Lories RJ. Spontaneous arthritis and ankylosis in male DBA/1 mice: further evidence for a role of behavioral factors in “stress-induced arthritis” Biol Proced Online. 2012;14:10. [PMC free article] [PubMed] [Google Scholar] 38. Lories RJ, Derese I, Ceuppens JL, Luyten FP. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 2003;48:2807–18. [PubMed] [Google Scholar] 39. Wellcome Trust Case Control Consortium, Australo-Anglo-American Spondylitis Consortium (TASC) Burton PR, Clayton DG, Cardon LR, Craddock N, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–37. [PMC free article] [PubMed] [Google Scholar] 40. Reveille JD. Genetics of spondyloarthritis-beyond the MHC. Nat Rev Rheumatol. 2012;8:296–304. [PubMed] [Google Scholar] 41. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med. 2012;18:1069–76. [PubMed] [Google Scholar] 42. Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Canete JD, et al. Interleukin-17–positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012;64:99–109. [PubMed] [Google Scholar] 43. Appel H, Maier R, Wu P, Scheer R, Hempfing A, Kayser R, et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res Ther. 2011;13:R95. [PMC free article] [PubMed] [Google Scholar] 44. Lories RJ, McInnes I. Primed for inflammation: enthesis resident cells. Nat Med. 2012;18:1018–9. [PubMed] [Google Scholar] 45. Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, et al. β-glucan triggers spondylarthritis and Crohn’s disease–like ileitis in SKG mice. Arthritis Rheum. 2012;64:2211–22. [PubMed] [Google Scholar] 46. Rehaume LM, Mondot S, Aguirre de Carcer D, Velasco J, Benham H, Hasnain SZ, et al. ZAP-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in SKG mice. Arthritis Rheumatol. 2014;66:2780–92. [PubMed] [Google Scholar] 47. Benham H, Rehaume LM, Hasnain SZ, Velasco J, Baillet AC, Ruutu M, et al. Interleukin-23 mediates the intestinal response to microbial β-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol. 2014;66:1755–67. [PubMed] [Google Scholar] 48. Yamamoto M, Nakajima K, Takaishi M, Kitaba S, Magata Y, Kataoka S, et al. Psoriatic inflammation facilitates the onset of arthritis in a mouse model. J Invest Dermatol. 2015;135:445–53. [PubMed] [Google Scholar] 49. Khmaladze I, Kelkka T, Guerard S, Wing K, Pizzolla A, Saxena A, et al. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc Natl Acad Sci U S A. 2014;111:E3669–78. [PMC free article] [PubMed] [Google Scholar] 50. Tan AL, Benjamin M, Toumi H, Grainger AJ, Tanner SF, Emery P, et al. The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis—a high-resolution MRI and histological study. Rheumatology (Oxford) 2007;46:253–6. [PubMed] [Google Scholar] 51. Naredo E, Moller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E, et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford) 2011;50:1838–48. [PubMed] [Google Scholar] 52. Yasser R, Yasser E, Hanan D, Rasker JJ. Enthesitis in seronegative spondyloarthropathies with special attention to the knee joint by MRI: a step forward toward understanding disease pathogenesis. Clin Rheumatol. 2011;30:313–22. [PubMed] [Google Scholar] 53. Weiss P, Beukelman T, Schanberg LE, Kimura Y, Colbert RA CARRAnet Investigators. Enthesitis is a significant predictor of decreased quality of life, function, and arthritis-specific pain across juvenile idiopathic arthritis (JIA) categories: preliminary analyses from the CARRAnet registry. Arthritis Rheum. 2011;63(Suppl):S105. [Google Scholar] 54. Weiss PF. Evaluation and treatment of enthesitis-related arthritis. Curr Med Lit Rheumatol. 2013;32:33–41. [PMC free article] [PubMed] [Google Scholar] 55. D’Agostino MA, Olivieri I. Enthesitis. Best Pract Res Clin Rheumatol. 2006;20:473–86. [PubMed] [Google Scholar] 56. D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–33. [PubMed] [Google Scholar] 57. D’Agostino MA, Aegerter P, Bechara K, Salliot C, Judet O, Chimenti MS, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis. 2011;70:1433–40. [PubMed] [Google Scholar] 58. Ward MM, Reveille JD, Learch TJ, Davis JC, Jr, Weisman MH. Occupational physical activities and long-term functional and radiographic outcomes in patients with ankylosing spondylitis. Arthritis Rheum. 2008;59:822–32. [PMC free article] [PubMed] [Google Scholar] 59. Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann Rheum Dis. 2015;74:437–43. [PMC free article] [PubMed] [Google Scholar] 60. Wiell C, Norregaard J, Szkudlarek M, Hasselquist M, Moller JM, Terslev L, et al. Ultrasonography of finger joints, tendons and entheses in patients with spondyloarthropathy: a comparison with clinical examination and MRI. Ann Rheum Dis. 2005;64(Suppl III):327. [Google Scholar] 61. Wiell C, Norregaard J, Szkudlarek M, Hasselquist M, Moller JM, Terslev L, et al. Ultrasonography of lower extremity tendons and entheses in patients with spondyloarthropathy: a comparison with clinical examination and MRI. Ann Rheum Dis. 2005;64(Suppl III):378. [Google Scholar] 62. Amor B, Dougados M, Mijiyawa M. Criteria for the classification of spondylarthropathies. Rev Rhum Mal Osteoartic. 1990;57:85–9. In French. [PubMed] [Google Scholar] 63. Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. the European Spondylarthropathy Study Group. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–27. [PubMed] [Google Scholar] 64. Mander M, Simpson JM, McLellan A, Walker D, Goodacre JA, Dick WC. Studies with an enthesis index as a method of clinical-assessment in ankylosing spondylitis. Ann Rheum Dis. 1987;46:197–202. [PMC free article] [PubMed] [Google Scholar] 65. Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, Landewe R, van ver Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis. 2003;62:127–32. [PMC free article] [PubMed] [Google Scholar] 66. Klauser AS, Wipfler E, Dejaco C, Moriggl B, Duftner C, Schirmer M. Diagnostic values of history and clinical examination to predict ultrasound signs of chronic and acute enthesitis. Clin Exp Rheumatol. 2008;26:548–53. [PubMed] [Google Scholar] 67. Braun J, Bollow M, Eggens U, Konig H, Distler A, Sieper J. Use of dynamic magnetic resonance imaging with fast imaging in the detection of early and advanced sacroiliitis in spondylarthropathy patients. Arthritis Rheum. 1994;37:1039–45. [PubMed] [Google Scholar] 68. McGonagle D, Marzo-Ortega H, O’Connor P, Gibbon W, Pease C, Reece R, et al. The role of biomechanical factors and HLA–B27 in magnetic resonance imaging–determined bone changes in plantar fascia enthesopathy. Arthritis Rheum. 2002;46:489–93. [PubMed] [Google Scholar] 69. Marzo-Ortega H, McGonagle D, O’Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum. 2001;44:2112–7. [PubMed] [Google Scholar] 70. Aquino MR, Tse SML, Gupta S, Rachlis AC, Stimec J. Whole-body MRI of juvenile spondyloarthritis: protocols and pictorial review of characteristic patterns. Pediatr Radiol. 2015;45:754–62. [PubMed] [Google Scholar] 71. Poggenborg RP, Eshed I, Ostergaard M, Sorensen IJ, Moller JM, Madsen OR, et al. Enthesitis in patients with psoriatic arthritis, axial spondyloarthritis and healthy subjects assessed by ‘head-to-toe’ whole-body MRI and clinical examination. Ann Rheum Dis. 2014;74:823–9. [PubMed] [Google Scholar] 72. Bandinelli F, Prignano F, Bonciani D, Bartoli F, Collaku L, Candelieri A, et al. Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Clin Exp Rheumatol. 2013;31:219–24. [PubMed] [Google Scholar] 73. Ruta S, Gutierrez M, Pena C, Garcia M, Arturi A, Filippucci E, et al. Prevalence of subclinical enthesopathy in patients with spondyloarthropathy: an ultrasound study. J Clin Rheumatol. 2011;17:18–22. [PubMed] [Google Scholar] 74. Feydy A, Lavie-Brion MC, Gossec L, Lavie F, Guerini H, Nguyen C, et al. Comparative study of MRI and power Doppler ultrasonography of the heel in patients with spondyloarthritis with and without heel pain and in controls. Ann Rheum Dis. 2012;71:498–503. [PubMed] [Google Scholar] 75. De Miguel E, Munoz-Fernandez S, Castillo C, Cobo-Ibanez T, Martin-Mola E. Diagnostic accuracy of enthesis ultrasound in the diagnosis of early spondyloarthritis. Ann Rheum Dis. 2011;70:434–9. [PubMed] [Google Scholar] 76. De Miguel E, Falcao S, Castillo C, Plasencia C, Garcia M, Branco JC, et al. Enthesis erosion in spondyloarthritis is not a persistent structural lesion. Ann Rheum Dis. 2011;70:2008–10. [PubMed] [Google Scholar] 77. Naredo E, Batlle-Gualda E, Garcia-Vivar ML, Garcia-Aparicio AM, Fernandez-Sueiro JL, Fernandez-Prada M, et al. Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: response to therapy of entheseal abnormalities. J Rheumatol. 2010;37:2110–7. [PubMed] [Google Scholar] 78. D’Agostino MA, Breban M, Said-Nahal R, Dougados M. Refractory inflammatory heel pain in spondylarthropathy: a significant response to infliximab documented by ultrasound [letter] Arthritis Rheum. 2002;46:840–1. [PubMed] [Google Scholar] 79. Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Marker-Hermann E, Zeidler H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012;71:1616–22. [PubMed] [Google Scholar] 80. Kroon F, Landewe R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:1623–9. [PubMed] [Google Scholar] 81. Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70:896–904. [PMC free article] [PubMed] [Google Scholar] 82. Marzo-Ortega H, McGonagle D, Jarrett S, Haugeberg G, Hensor E, O’Connor P, et al. Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann Rheum Dis. 2005;64:1568–75. [PMC free article] [PubMed] [Google Scholar] 83. Baraliakos X, Davis J, Tsuji W, Braun J. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor α receptor fusion protein etanercept. Arthritis Rheum. 2005;52:1216–23. [PubMed] [Google Scholar] 84. Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150–7. [PMC free article] [PubMed] [Google Scholar] 85. Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA M02-570 Study Group. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy [published erratum appears in J Rheumatol 2007;34:1439] J Rheumatol. 2007;34:1040–50. [PubMed] [Google Scholar] 86. Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four–week efficacy and safety results of a randomized, placebo-controlled study [published erratum appears in Arthritis Rheum 2010;62:2555] Arthritis Rheum. 2009;60:976–86. [PubMed] [Google Scholar] 87. Van der Heijde D, Schiff MH, Sieper J, Kivitz AJ, Wong RL, Kupper H, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis. 2009;68:922–9. [PMC free article] [PubMed] [Google Scholar] 88. Olivieri I, Giasi V, Scarano E, Gigliotti P, D’Angelo S, Padula A. A brief course of anti-TNF-α therapy can cure recurrent episodes of HLA-B27-associated severe and refractory heel enthesitis [letter] Clin Exp Rheumatol. 2009;27:1057. [PubMed] [Google Scholar] 89. Hreggvidsdottir HS, Noordenbos T, Baeten DL. Inflammatory pathways in spondyloarthritis. Mol Immunol. 2014;57:28–37. [PubMed] [Google Scholar] 90. McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–9. [PubMed] [Google Scholar] 91. McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, improves active psoriatic arthritis: 24-week efficacy and safety data from a phase 3 randomized, multicenter, double-blind, placebo-controlled study using subcutaneous dosing [abstract] Arthritis Rheumatol. 2014;66:3529. [Google Scholar] 92. Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479–88. [PubMed] [Google Scholar] 93. Maksymowych WP, Lambert R, Jhangri GS, Leclercq S, Chiu P, Wong B, et al. Clinical and radiological amelioration of refractory peripheral spondyloarthritis by pulse intravenous pamidronate therapy. J Rheumatol. 2001;28:144–55. [PubMed] [Google Scholar] 94. Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 2012;51:1368–77. [PMC free article] [PubMed] [Google Scholar] 95. Kaltwasser JP, Nash P, Gladman D, Rosen CF, Behrens F, Jones P, et al. for the Treatment of Psoriatic Arthritis Study Group. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2004;50:1939–50. [PubMed] [Google Scholar] 96. Clegg DO, Reda DJ, Mejias E, Cannon GW, Weisman MH, Taylor T, et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis: a Department of Veterans Affairs Cooperative Study. Arthritis Rheum. 1996;39:2013–20. [PubMed] [Google Scholar] 97. Song IH, Heldmann F, Rudwaleit M, Haibel H, Weiss A, Braun J, et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis. 2011;70:1108–10. [PubMed] [Google Scholar] 98. Lekpa FK, Poulain C, Wendling D, Soubrier M, De Bandt M, Berthelot JM, et al. Is IL-6 an appropriate target to treat spondyloarthritis patients refractory to anti-TNF therapy? A multicentre retrospective observational study. Arthritis Res Ther. 2012;14:R53. [PMC free article] [PubMed] [Google Scholar] 99. Song IH, Heldmann F, Rudwaleit M, Listing J, Appel H, Haug-Rost I, et al. One-year follow-up of ankylosing spondylitis patients responding to rituximab treatment and re-treated in case of a flare. Ann Rheum Dis. 2013;72:305–6. [PubMed] [Google Scholar] 100. Wendling D, Dougados M, Berenbaum F, Brocq O, Schaeverbeke T, Mazieres B, et al. on behalf of the French Society of Rheumatology and the Club Rhumatismes et Inflammation. Rituximab treatment for spondyloarthritis. A nationwide series: data from the AIR registry of the French Society of Rheumatology. J Rheumatol. 2012;39:2327–31. [PubMed] [Google Scholar]

|
Scooped by
Gilbert C FAURE
February 20, 2020 1:20 PM
|
Despite the introduction of numerous biologic agents for the treatment of rheumatoid arthritis (RA) and other forms of inflammatory arthritis, low-dose methotrexate therapy remains the gold standard in RA therapy. Methotrexate is generally the first-line drug for the treatment of RA, psoriatic arthritis and other forms of inflammatory arthritis, and it enhances the effect of most biologic agents in RA. Understanding the mechanism of action of methotrexate could be instructive in the appropriate use of the drug and in the design of new regimens for the treatment of RA. Although methotrexate is one of the first examples of intelligent drug design, multiple mechanisms potentially contribute to the anti-inflammatory actions of methotrexate, including the inhibition of purine and pyrimidine synthesis, transmethylation reactions, translocation of nuclear factor-κB (NF-κB) to the nucleus, signalling via the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway and nitric oxide production, as well as the promotion of adenosine release and expression of certain long non-coding RNAs. Methotrexate can suppress inflammation via multiple mechanisms that can differ across different cell types. Understanding these mechanisms might enable better understanding of the disease and prediction of treatment responses.

|
Rescooped by
Gilbert C FAURE
from Osteoporosis New drugs Review
December 20, 2019 4:55 AM
|
Abstract
Current osteoporosis medications reduce fractures significantly but have rare and serious adverse effects (osteonecrosis of the jaw, atypical femoral fractures) that may limit their safety for long-term use. Insights from basic bone biology and genetic disorders have led to recent advances in therapeutics for osteoporosis. New approaches now in clinical use include the antisclerostin monoclonal antibody romosozumab, as well as the parathyroid hormone–related peptide analog abaloparatide. Clinical trial data show significant antifracture benefits with recently approved romosozumab. Studies using abaloparatide build on our longstanding experience with teriparatide and the importance of consolidating the bone mineral density gains achieved from an anabolic agent by following it with an antiresorptive. Combination and sequential treatments using osteoporosis medications with different mechanisms of action have also been tested with promising results. On the horizon is the potential for cell-based therapies (e.g., mesenchymal stem cells) and drugs that target the elimination of senescent cells in the bone microenvironment.
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
October 23, 2019 4:46 AM
|
KEY POINTS TCR-γδ tetramer identified ligand expression by flow cytometry. TCR-γδ ligands were induced on activated monocytes or T cells. Bioinformatics combined with mass spectrometry produced an overlapping list of 16 candidate ligands. Visual Abstract Abstract Lack of understanding of the nature and physiological regulation of γδ T cell ligands has considerably hampered full understanding of the function of these cells. We developed an unbiased approach to identify human γδ T cells ligands by the production of a soluble TCR-γδ (sTCR-γδ) tetramer from a synovial Vδ1 γδ T cell clone from a Lyme arthritis patient. The sTCR-γδ was used in flow cytometry to initially define the spectrum of ligand expression by both human tumor cell lines and certain human primary cells. Analysis of diverse tumor cell lines revealed high ligand expression on several of epithelial or fibroblast origin, whereas those of hematopoietic origin were largely devoid of ligand. This allowed a bioinformatics-based identification of candidate ligands using RNAseq data from each tumor line. We further observed that whereas fresh monocytes and T cells expressed low to negligible levels of TCR-γδ ligands, activation of these cells resulted in upregulation of surface ligand expression. Ligand upregulation on monocytes was partly dependent upon IL-1β. The sTCR-γδ tetramer was then used to bind candidate ligands from lysates of activated monocytes and analyzed by mass spectrometry. Surface TCR-γδ ligand was eliminated by treatment with trypsin or removal of glycosaminoglycans, and also suppressed by inhibition of endoplasmic reticulum–Golgi transport. Of particular interest was that inhibition of glycolysis also blocked TCR-γδ ligand expression. These findings demonstrate the spectrum of ligand(s) expression for human synovial Vδ1 γδ T cells as well as the physiology that regulates their expression. This article is featured in In This Issue, p.2353 Introduction Full understanding of γδ T cell biology has been handicapped by ignorance of the ligands for most TCR-γδ. γδ T cells reside at mucosal and epithelial barriers and often accumulate at sites of inflammation with autoimmunity, infections, or tumors (1). Evidence suggests that γδ T cells provide protection against infections with bacteria, viruses, and protozoans and are generally beneficial in autoimmunity (1–17). In addition, a role for γδ T cells in the immune response against tumors in humans is evident from a seminal study reporting that intratumoral γδ T cells are the most favorable prognostic immune population across 39 cancer types in humans (18). γδ T cells are often highly lytic against transformed proliferative cells, infected cells, and infiltrating CD4+ T cells in inflammatory arthritis (9, 17, 19). They can produce a variety of cytokines including IFN-γ, TNF-α, and IL-17 (20), as well as insulin-like growth factor-1 (IGF1) and keratinocyte growth factor (KGF) that promote epithelial wound repair (21). These collective studies indicate that a principal function of γδ T cells is in response to tissue injury of various causes. It is, thus, not surprising that γδ T cells are often suggested to react to host components that are upregulated or exposed during proliferation or cell injury (22). As such, γδ T cells may function in tissue homeostasis and immunoregulation as much as in protection from infection. Yet in the vast majority of cases, little if anything is known regarding the nature of these self-components or whether they actually engage the TCR-γδ. Whereas αβ T cells recognize proteins that are processed into peptides and presented on MHC molecules, the few proposed ligands for γδ T cells suggest that they recognize mostly intact proteins directly, without MHC restriction. This makes them highly attractive for immunotherapy. Despite the elaborate mechanisms that αβ T cells and B cells use to prevent autoreactivity, γδ T cells have been frequently reported to respond to autologous proteins. Furthermore, in contrast to other lymphocytes that maximize the potential diversity of their receptors, γδ T cells frequently show limitations in their diversity. Thus, human γδ T cells comprise a subset of Vδ2 T cells, the predominant γδ in peripheral blood that respond to prenyl phosphates and certain alkyl amines (23–25), and Vδ1 T cells, which do not respond to these compounds and often accumulate at epithelial barriers and sites of inflammation (1). A similar limited repertoire occurs in the mouse in which Vγ5Vδ1 cells colonize the epidermis, and a Vγ6Vδ1 subset colonizes the tongue, lung, and female reproductive tract (21, 26). This restricted repertoire implies that TCR-γδ ligands may also be limited. This may provide for a more rapid response and perhaps explain why, in contrast to αβ T cells and B cells, it is difficult to generate Ag-specific γδ T cells by immunization with a defined Ag. Various ligands for γδ T cells have been proposed, although only a few have been confirmed to bind to TCR-γδ, and these lack any obvious similarity in structure. γδ T cells for which ligands have been identified include the murine γδ T cell clone G8, which recognizes the MHC class I–like molecules T10 and T22 (27), γδ T cells from mice infected with HSV that recognize herpes glycoprotein gl (28), a subset of murine and human γδ T cells that bind the algae protein PE (20), a human γδ T cell clone G115 that recognizes ATP synthase complexed with ApoA-1 (28), a human γδ T cell clone (Vγ4Vδ5) from a CMV-infected transplant patient that recognizes endothelial protein C receptor (EPCR) (29), and some human Vδ1 T cells that recognize CD1d-sulfatide Ags (30). However, to date no systematic process has been reported for determining the spectrum of human TCR-γδ ligands. To provide an unbiased approach for the identification of candidate ligands for human γδ T cells, we produced a biotinylatable form of a soluble TCR-γδ (sTCR-γδ) from a synovial Vδ1 γδ T cell clone of a Lyme arthritis patient. The tetramerized sTCR-γδ was used in flow cytometry to identify various cell types that expressed candidate ligands. Initial analysis of 24 tumor cell lines identified a set of nine ligand-positive tumors, enriched for those of epithelial and fibroblast origin, and 15 ligand-negative tumors, largely of hematopoietic origin. In addition, ligand was not expressed by primary monocytes or T cells, although each could be induced to express ligand following their activation. Ligand expression was sensitive to trypsin digestion, revealing the protein nature of the ligands, and was also reduced by inhibition of glycolysis. These findings provide a framework and strategy for the identification of individual ligands for human synovial γδ T cells. Materials and Methods Production of a sTCR-γδ Human synovial γδ T cell clones from a Lyme arthritis patient were produced as previously described (9, 31). One of these clones, Bb15, was chosen for production of the sTCR-γδ using modification of a previously reported procedure (32, 33). Both TCR chains were produced as a single transcript in a baculovirus vector. The pBACp10pH vector used contains two back-to-back promoters, p10 and polyhedrin (Fig. 1A). The p10 promoter is followed by multiple cloning sites for the γ-chain, and the polyhedrin promoter is followed by multiple cloning sites for the δ-chain. Downstream of the γ-chain, we placed a hexa-His tag for nickel column purification, followed by a biotinylation sequence for tetramerization. The γ-chain and δ-chain were PCR amplified using high fidelity polymerase (Deep Vent Polymerase; New England Biolabs). Both TCR chain sequences were verified following the initial PCR amplification as well as after insertion into the pBACp10pH vector. Virus encoding the sTCR-γδ was generated by cotransfection of Sf21 moth cells using the Sapphire baculovirus DNA and Transfection kit (Orbigen) with the sTCR pBACp10pH construct. Virus was harvested 6 d later and used as primary stocks (P1 stock). Two additional rounds of viral amplification, P2 and P3, were completed using midlog phase Sf21 cells (∼1.6 × 106 cells/ml) allowed to adhere for 1 h before infecting at a multiplicity of infection of 0.01 or 0.1 with P1 and P2 stock, respectively. After 72 h of infection, culture medium was clarified by centrifugation (1000 × g for 10 min) and filtration (VacuCap 90PF 0.8/0.2 μm Supor membrane filter units; Pall, Westborough, MA) before storing in the dark at 4°C until use. Protein production occurred in 12-l batches of midlog phase (∼1.6 × 106 cells/ml) Hi5 cells growing in suspension (0.5 l of culture in 1 l spinner flasks) and infected with P3 stock at a 1:50 dilution. Following 72 h of infection, cells were removed by centrifugation and filtration as described above. The filtered supernatant (∼12 l) containing secreted sTCR-γδ was concentrated to ∼100 ml before dialyzing against 1 l of nickel column loading buffer (20 mM NaPhosphate buffer, pH 7.4, 20 mM imidazole, 0.5 M NaCl) using a Pellicon diafiltration system with two 10K MWCO membranes (MilliporeSigma, Burlington, MA) back down to ∼100 ml. After system flushing, the final sample volume was ∼200 ml. It was then loaded onto loading buffer–equilibrated His-Trap HP columns (GE Healthcare, Little Chalfont, U.K.) at 100 ml per 2 × 5 ml columns. Columns were washed with at least 10 column volumes of loading buffer until baseline absorption was achieved. Bound proteins were eluted using a gradient from 20 to 500 mM imidiazole over 20 column volumes. Elution was monitored by absorbance at 280 nM, and 1 ml fractions were collected. Fractions containing the target protein were identified using SDS-PAGE gel analysis using Coomassie Blue. High purity (>95%) sTCR-γδ fractions were pooled, dialyzed against PBS (pH 7.4), and frozen at −80°C until used in future studies. Yields were typically ∼1.0–2.5 mg/l of culture. Purified sTCR-γδ was then biotinylated using a biotin-protein ligase system (Avidity) and tetramerized with streptavidin-PE (BioLegend) for FACS staining. Verification of TCR-γδ protein was confirmed by SDS-PAGE gel analysis using Coomassie Blue as well as immunoblot using Abs to Vδ1 or Cγ (Endogen). Flow cytometry Cells were stained with either sTCR-γδ-PE (10 μg/ml) or negative controls that included streptavidin-PE (10 μg/ml), IgG-PE (10 μg/ml) (BioLegend), or a sTCRαβ-PE (a kind gift of Dr. M. Davis). Additional surface staining of T cells consisted of CD4, CD8, CD19, and CD25 (BioLegend). Live–Dead staining (BD Bioscience) was used to eliminate dead cells from analysis. Samples were run on an LSRII flow cytometer (Becton Dickinson). Purification and activation of human monocytes and T cells and cell lines Human monocytes were purified from human PBMC using CD14-labeled magnetic beads, followed by column purification (Miltenyi Biotec) and then cultured in RPMI complete medium with 10% FCS in the absence or presence of either a Borrelia burgdorferi sonicate (10 μg/ml) or LPS (1 μg/ml; Sigma-Aldrich) for 18 h. To some cultures were added TNF-α (10 ng/ml) (BioLegend), anti-TNF-α (10 μg/ml) (BioLegend), IL-1β (10 pg/ml) (Invitrogen), or anti–IL-1β (5 μg/ml) (R&D Systems). Cells were then stained with the sTCR-γδ tetramer. T cells from PBMC were either used fresh or were activated with anti-CD3/anti-CD28 (each 10 μg/ml; BioLegend) + IL-2 (50 U/ml; Cetus) and propagated for 3 d. Cells were then stained with the sTCR-γδ tetramer. Human PBMC were obtained using an approved protocol from The University of Vermont Human Studies Committee. Verified cell lines were obtained from American Type Culture Collection. CHO cells deficient for glycosaminoglycans (GAGs) were derived as previously described (34). Bioinformatics analysis Expression profiling (35) based on Illumina RNAseq technology (36) was used to characterize the transcriptomes of 22 of the 24 tumor cell lines examined (excluding bronchoepithelial cell line and 2fTGH). Expression data for all known genes (37) were generated, and those genes whose representation in tetramer-positive cell lines was significantly higher than in negative cell lines were considered as candidate ligands. Mass spectrometry analysis Biotinylated sTCR-γδ was bound to avidin magnetic beads and then incubated with cell lysates from monocytes activated with B. burgdorferi sonicate. Magnetic beads alone, without TCR-γδ tetramer, with monocyte lysates served as a negative control. After 4 h, beads were washed five times, and bound proteins were then separated on polyacrylamide gels. Gel lanes for each sample type were cut into 12 identical regions and diced into 1-mm cubes. In-gel tryptic digestion was conducted on each region as previously described (38). Extracted peptides were subjected to liquid chromatography tandem mass spectrometry (38), except that the analysis was performed using an LTQ linear ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Tandem mass spectra were searched against the forward and reverse concatenated human IPI database using SEQUEST, requiring fully tryptic peptides, allowing a mass tolerance of 2 Da and mass additions of 16 Da for the oxidation of methionine and 71 Da for the addition of acrylamide to cysteine. SEQUEST matches in the first position were then filtered by XCorr scores of 1.8, 2, and 2.7 for singly, doubly, and triply charged ions, respectively. Protein matches made with more than two unique peptides were further considered. This list had a peptide false discovery rate of <0.01%. Inhibition of glycolysis, transcription, translation, and endoplasmic reticulum–Golgi transport or trypsin or heparinases I–III treatment Inhibition of glycolysis was performed using the 2-deoxyglucose (2-DG, 5 mM; Sigma-Aldrich) for 48 h. Transcription and translation were inhibited using, respectively, actinomycin D (5 μg/ml; ICN Biomedicals) or cycloheximide (1 μg/ml; MilliporeSigma) for 18 h. Endoplasmic reticulum (ER)–Golgi transport was blocked using brefeldin A (1:1000) or monensin (1:1400) (BD Bioscience) for 18 h. Cell surface protein digestion was performed using trypsin (Invitrogen) (1×; 5–10 min, 37°C.). GAGs were removed from cells by treatment with heparinases I–III (2 μU/ml) for 30 min in RPMI 1640 with no serum. The reaction was then stopped by the addition of PBS–BSA. Statistical analysis The following statistical tests were used: unpaired Student t test when comparing two conditions, and one-way ANOVA with Sidak test for correction for multiple comparisons when comparing multiple variables across multiple conditions. Results Production of a human synovial sTCR-γδ We previously produced a panel of synovial Vδ1 γδ T cells from Lyme arthritis patients (9, 31). A representative clone, Bb15 (Vδ1Vγ9), was selected from which to clone its TCR-γδ. The pBACp10pH vector has been used previously to produce murine sTCR-γδ tetramers (33). It contains two back-to-back promoters, p10 and polyhedrin, in which the p10 promoter is followed by multiple cloning sites for inserting the γ-chain, and the polyhedrin promoter is followed by multiple cloning sites for inserting the δ-chain (Fig. 1A). Downstream of the γ-chain we placed a hexa-His tag for purification, followed by a biotinylation BRP sequence for tetramerization with streptavidin-PE. Protein production was undertaken in Hi5 cells followed by purification using His-Trap HP columns. Fractions were analyzed by SDS-PAGE, and those with protein of the correct size were pooled, with yields typically of 1–2 mg/l of culture. A sample sTCR-γδ preparation is shown in Fig. 1B, stained with Coomassie Blue, showing bands of the expected size for the heterodimer under nonreducing (59 kDa) and reducing conditions (30/28 kDa for the γ- and δ-chains, respectively). The protein was stained by immunoblot with Abs to either Vδ1 or Cγ (Fig. 1C) and also blocked anti-γδ Ab staining of the synovial γδ T cell clones (Fig. 1D). The purified sTCR-γδ was then biotinylated and tetramerized with streptavidin-PE for use by flow cytometry. As an additional measure of specificity, sTCR-γδ tetramer staining of a fibrosarcoma tumor cell line (2fTGH) could be inhibited by anti-γδ Ab but not control IgG (Fig. 1E). Finally, staining of 2fTGH cells with the sTCR-γδ tetramer was dose dependent but did not increase with increasing dose on a negative tumor line, Daudi (Fig. 1F). FIGURE 1. Production of human synovial sTCR-γδ. (A) pBACp10pH vector containing the δ-chain driven by the polyhedrin promoter and the γ-chain with hexa-His and biotinylation BRP sequences driven by the p10 promoter from γδ T cell clone Bb15 (Vγ9Vδ1). (B) Sample of nickel NTA column-purified sTCR-γδ analyzed by SDS-PAGE under reducing and nonreducing conditions, and stained with Coomassie Blue. (C) Immunoblot of sTCR-γδ stained with anti-Vδ1 or anti-Cγ. (D) γδ T cell clone Bb15 was stained with anti–TCR-γδ Ab in the absence or presence of competing sTCR-γδ. (E) The fibrosarcoma cell line 2fTGH was stained with the sTCR-γδ in the absence or presence of the indicated concentrations of anti-γδ Ab or control IgG. (F) Titration of sTCR-γδ staining of the positively staining tumor line 2fTGH or negatively staining line Daudi. Number inserts indicate percent positively staining cells. Findings are representative of three experiments. Expression of sTCR-γδ candidate ligand(s) varies among cell lines We initially used the sTCR-γδ tetramer to screen a panel of 24 cell lines from a variety of cell types. None of the cell lines stained with the negative controls (IgG-PE, avidin-PE, or sTCR-αβ tetramer-PE), but the sTCR-γδ tetramer gave a spectrum of staining in which nine cell lines were strongly positive and the other cell lines manifested low to undetectable surface staining (Fig. 2). Of interest was that the positive group was enriched for cell lines of epithelial and fibroblast origin, cell types known to exist where γδ T cells are often found, such as skin, intestines, and synovium. With this information, expression profiling (35) using available RNAseq was used to characterize the transcriptomes of 22 of the 24 tumor cell lines (RNAseq on the bronchoepithelial and 2fTGH were not available). Expression data for all known genes (37) were generated, and those genes whose representation in tetramer-positive cell lines was significantly higher than in negative cell lines were considered to be candidate ligands. This produced an initial list of candidate ligands for sTCR-γδ (Supplemental Table I). FIGURE 2. sTCR-γδ tetramer staining of a cell line panel. A panel of 24 diverse cell lines was stained with either sTCR-αβ or sTCR-γδ, gated on live cells, and examined by flow cytometry. Shown are examples of tumors representing either (A) positive staining or (B) negative staining with sTCR-γδ, with the complete list summarized below each example. Number inserts indicate mean fluorescence intensity of entire histogram. Findings are representative of four experiments. Candidate sTCR-γδ ligands are sensitive to trypsin and reduced by inhibition of transcription, translation, ER–Golgi transport, or removal of GAGs We treated the positively staining cell lines with trypsin and noted a complete disappearance of surface staining, as exemplified for bronchoepithelial cells in Fig. 3A. Similar results were observed with two additional tumor lines. This supports the view that the TCR-γδ ligand contains a protein component essential for recognition by the receptor. We also observed no increase in sTCR-γδ tetramer staining of cells (C1R or HeLa) expressing CD1a, b, c, or d, nor with MICA/B (data not shown). Thus, at present there is no evidence that the synovial Vδ1 TCR-γδ ligand is one of these MHC class I–like molecules, at least bound to endogenous molecules from these particular cell lines. FIGURE 3. sTCR-γδ ligand is sensitive to protease, blockers of ER–Golgi transport, translation, or transcription and contains GAGs. The human bronchoepithelial cell line was either untreated or treated with (A) trypsin for 15 min, (B) untreated or treated for 18 h with cycloheximide or actinomycin D, or (C) untreated or treated for 18 h with brefeldin A or monensin. Cells were then stained with sTCR-γδ tetramer. (D) The 2fTGH fibrosarcoma cell line, wild-type CHO cells, or GAG-deficient CHO cells were either untreated or treated with a combination of heparinases I–III for 30 min and then stained with sTCR-γδ tetramer. Number inserts indicate mean fluorescence intensity of entire histogram. Findings are representative of three experiments. We further determined that surface TCR-γδ ligand expression was reduced by inhibition of protein translation or transcription with, respectively, cycloheximide or actinomycin D (Fig. 3B). Surface ligand was also considerably reduced by inhibition of transport from the ER to Golgi using either brefeldin A or monensin (Fig. 3C). This further demonstrated the protein nature of candidate TCR-γδ ligands. Finally, we examined the extent to which GAGs contribute to ligand binding by TCR-γδ. This was tested in two ways. Initially, the ligand-positive fibrosarcoma cell line 2fTGH was either treated or not with heparinases I–III, which removes most GAGs. This considerably reduced sTCR-γδ tetramer staining (Fig. 3D). This was further supported by the observation that sTCR-γδ stained wild-type but not GAG-deficient CHO cells (Fig. 3D). sTCR-γδ ligands are expressed by activated monocytes In considering what primary cells might express ligand(s) for the sTCR-γδ, we first examined fresh monocytes, as we had observed previously that following their activation with B. burgdorferi or LPS, monocytes could activate the synovial γδ T cell clones (31). Consistent with these earlier findings, we observed that the sTCR-γδ tetramer did not stain freshly isolated human monocytes, but following 24 h activation with a sonicate of B. burgdorferi or LPS, there was a robust upregulation of sTCR-γδ tetramer staining (Fig. 4). The same cells did not stain with negative controls that included avidin-PE, IgG-PE, or a human sTCR-αβ tetramer-PE. Because activated monocytes are known to produce certain cytokines, particularly TNF-α and IL-1β, we examined the possible influence of these cytokines on ligand expression. Curiously, the low level of sTCR-γδ tetramer staining of fresh monocytes was reduced further with TNF-α, whereas ligand expression by Borrelia-activated monocytes was not affected by the further addition of TNF-α or blocking anti–TNF-α Ab (Fig. 4B). By contrast, IL-1β increased ligand expression by fresh but not activated monocytes, and blocking anti–IL-1β Ab partially inhibited ligand expression by activated monocytes (Fig. 4C). Thus, sTCR-γδ ligand expression appears to be partly regulated by certain monocyte-derived cytokines. FIGURE 4. TCR-γδ ligand is induced on human monocytes following activation. (A) Freshly isolated monocytes were either unstimulated or activated with B. burgdorferi or LPS for 18 h and then stained with the indicated reagents and analyzed by flow cytometry. (B and C) Fresh monocytes or monocytes activated with Borrelia were incubated in the presence of medium alone or TNF-α or blocking anti-TNF-α (B) or IL-1β or blocking anti–IL-1β (C). Number inserts indicate percent positively staining cells. Error bars represent SEM. Findings are representative of four experiments. Given the induction of sTCR-γδ ligand expression by activated monocytes, we prepared lysates from Borrelia-activated monocytes and then used the biotinylated sTCR-γδ complexed with avidin magnetic beads as a bait. Following incubation with the monocyte lysates, the sTCR-γδ was isolated by magnetic purification and washed five times; bound proteins were separated on polyacrylamide gels, and gel slices were subjected to trypsin digestion and analyzed by mass spectrometry. Avidin magnetic beads alone incubated with monocyte lysates served as a negative control. This analysis yielded 291 unique proteins (Supplemental Table II). When compared with the list produced by the RNAseq bioinformatics approach of the tumor lines, 16 proteins were found in common (Supplemental Table III). Of interest is that two of these, Annexin A2 and heat shock protein 70, have previously been proposed as γδ ligands (39–41). sTCR-γδ ligands are expressed by activated T cells We further analyzed freshly isolated PBL from three individuals of various ages (28–66). This consistently revealed that fresh CD8+ T cells exhibited negligible sTCR-γδ staining, whereas a subset of fresh CD4+ T cells manifested modest levels of sTCR-γδ staining (Fig. 5A). In contrast to the freshly isolated T cells, following 3 d activation with anti-CD3/CD28 + IL-2, we observed that a subset of both CD4+ and CD8+ T cells now displayed high levels of sTCR-γδ staining (Fig. 5B). Both the proportion of cells expressing ligand and the density was higher on activated CD4+ T cells compared with CD8+ T cells. Given that in vitro–activated proliferating T cells express sTCR-γδ ligand, we considered that the subset of fresh CD4+ T cells expressing ligand might also represent a proliferative subset. One of the most rapidly proliferative T cell subsets in vivo is T regulatory cells (Treg) (42). Treg can be identified as a subset of fresh CD4+ T cells expressing CD25. Indeed, when we subset fresh human CD4+ T cells based on CD25 expression, sTCR-γδ tetramer staining was again observed preferentially by the CD25+ subset (Fig. 5C). FIGURE 5. sTCR-γδ tetramer stains a subset of activated human T cells and Treg. PBL were stained with Abs to CD4 and CD8 as well as with sTCR-αβ tetramer-PE or sTCR-γδ tetramer-PE either (A) freshly isolated or (B) 3 d after activation with anti-CD3/CD28 + IL-2. Number inserts indicate the percentages of T cells staining negatively or positively with sTCR-γδ tetramer, as a portion of the total CD4+ or CD8+ subsets, as well as mean fluorescence intensity (MFI) in some cases. Findings are representative of six experiments. (C) Freshly isolated PBL were stained with anti-CD4, anti-CD25 or isotype control, and streptavidin-PE (SA-PE) or sTCR-γδ-PE. Shown are cells gated on CD4 expression. Number inserts indicate MFI of sTCR-γδ-PE staining for CD25+ and CD25− subsets. Findings are representative of two experiments. TCR-γδ ligand expression is partly dependent upon glycolysis The finding that fresh monocytes and T lymphocytes expressed low to negligible levels of sTCR-γδ ligand(s), but upregulated expression following activation, raised the possibility that this might reflect the known induction of glycolysis following activation of T cells, monocytes, or dendritic cells (43, 44) and the resultant synthetic capacity promoted by glycolysis (45). This notion is supported by the fact that ligand-expressing Treg are also highly glycolytic (42). We thus examined this question in two ways. First, we exposed activated T cells to 2-DG, an inhibitor of glycolysis. This reduced expression of both CD25 and sTCR-γδ ligand (Fig. 6A). Second, we distinguished between activated T cells on day 3 based on their expression of CD25, as this identifies cells responsive to IL-2 and are hence most glycolytic (45). As shown in Fig. 6B, CD25+ T cells expressed sTCR-γδ ligand whereas the CD25− subset was devoid of ligand expression. Of further note is that within the CD25+ subset, CD4+ T cells again expressed more ligand than CD8+ T cells (Fig. 6B). We extended this analysis to the ligand-positive tumor 2fTGH and observed that 2-DG also resulted in reduced ligand expression in these cells (Fig. 6C). FIGURE 6. TCR-γδ ligand expression parallels glycolysis. (A and B) PBL were activated with anti-CD3/CD28 + IL-2 in the absence or presence of 2-DG (5 mM). On day 3, cells were stained with Abs to CD4, CD8, CD25, and sTCR-γδ tetramer-PE. Shown in (A) are the levels of CD25 and TCR-γδ ligand without or with 2-DG. Shown in (B) is the expression of TCR-γδ ligand in CD4+ or CD8+ subsets based on surface CD25. (C) 2fTGH cells were cultured for 48 h in either regular medium or medium plus 2-DG (5 mM). Cells were then stained with TCR-αβ or TCR-γδ. Number inserts indicate mean fluorescence intensity (MFI) of sTCR-γδ-PE staining. Findings are representative of three experiments. Discussion To our knowledge, the current findings provide the first unbiased characterization of the spectrum of ligand expression for human synovial Vδ1 γδ T cells. The range of ligand expression may reflect the various locations and seemingly diverse functions attributed to γδ T cells. For example, ligand induction by B. burgdorferi– or LPS-activated monocytes parallels their known ability to activate synovial γδ T cell clones (9, 31). In addition, ligand expression by fresh CD4+ but not CD8+ T cells also correlates with our previous observations that Lyme arthritis synovial γδ T cells suppress by cytolysis the expansion of synovial CD4+ but not CD8+ T cells in response to B. burgdorferi (9). Finally, defining the spectrum of tumor cell types that express TCR-Vδ1 ligands may help explain which tumors contain Vδ1 γδ T cells and impact their effectiveness as immunotherapy. The collective findings are also most consistent with the view that γδ T cells respond to self-proteins as much as or possibly more than foreign proteins. Although these results were obtained using a sTCR-γδ tetramer from a single synovial γδ T cell clone, the fact that it shares a common Vδ1 chain found on most synovial γδ T cells (9), as well as γδ T cells found in intestinal epithelium (1, 10, 21), several tumors (18), and cells expanded in PBL following certain infections such as HIV (46, 47) and CMV (29), suggests the possibility that Vδ1 γδ T cells from these other sources may share a common physiology of ligand expression. Previous studies of ligands for murine and human γδ T cells have come largely from the identification of individual molecules that activate a specific γδ T cell clone (27–30). Although this has been successful in some instances, the current study applied a broader approach of using a sTCR-γδ tetramer in an unbiased fashion to identify the spectrum of ligand expression and how they are regulated. This approach also provided two independent methods by which to identify candidate ligands. One method used RNAseq transcriptome analysis from 22 tumor cell lines to match genes increased in positively staining tumors and decreased in negatively staining tumors. The second approach used the sTCR-γδ tetramer as a bait to bind ligands from lysates of activated monocytes and then identify the bound proteins by mass spectrometry. It is of considerable intertest that among these two sets of candidate ligands were 16 in common, two of which, Annexin A2 and heat shock protein 70, have been previously proposed as ligands for γδ T cells (39–41). By contrast, surface sTCR-γδ tetramer binding was eliminated by treatment with trypsin or removal of GAGs, and also suppressed by inhibition of ER–Golgi transport, suggesting the involvement of a combination of protein and GAGs in tetramer binding. Future studies will explore through knockdown and transfection methods whether any of the candidate ligands we have identified activate the original γδ T cell clone and the extent to which GAG/glycoprotein binding may or may not be a confounder. Although the findings thus far have not determined whether there is one or several synovial Vδ1 TCR-γδ ligands, they do provide a framework for understanding the distribution and regulation of ligand expression, which is critical for better understanding of γδ T cell biology. For example, γδ T cells have been implicated in the defense against a variety of infections (2–7), which is consistent with our finding that different TLR agonists induce TCR-γδ ligand expression on monocytes. Similar studies using a murine sTCR-γδ also found ligands induced with bacterial infection (21). In addition, γδ T cells have been found to generally alleviate various autoimmune models (12–15), which may be consistent with the expression of ligand by a subset of activated CD4+ T cells. The induction of TCR-γδ ligand expression by activation of primary monocytes or T cells, as well as ligand expression by a variety of highly proliferative tumor cell lines, suggested that the metabolic state of cells may influence their ability to express TCR-γδ ligands. Activation of monocytes and T cells is known to induce a metabolic switch to glycolysis to provide the synthetic capacity for proliferation (43, 44). In addition, Treg, which are known to be glycolytic in vivo (42), spontaneously expressed ligand. Moreover, most tumors are highly glycolytic, and the inhibition of glycolysis in these cells also reduced ligand expression. Collectively, these findings suggest that some γδ T cells may function to survey and regulate highly proliferative cells. It is of some interest that the cell lines bearing high levels of TCR-γδ ligand expression were enriched for those of epithelial and fibroblast origin, because Vδ1 γδ T cells are typically found at epithelial barriers, such as skin or intestinal epithelium, as well as in inflamed synovium, which is rich in fibroblasts (48). By contrast, sTCR-γδ ligand expression was noticeably absent from most cell lines of hematopoietic origin. The spectrum of cell line staining with the human synovial sTCR-γδ also bears considerable similarity to previous results using a murine sTCR-γδ, which strongly stained epithelial and fibroblast tumors, and less well tumors of hematopoietic origin (33). These same murine sTCR-γδ also stained macrophages activated by TLR2 or TLR4 stimuli, similar to our findings with monocytes activated by Borrelia or LPS (49). Furthermore, staining of macrophages by the murine sTCR-γδ was also not affected by the absence of β2-microgloublin, suggesting little or no contribution of ligand by classical or nonclassical MHC class I molecules. This agrees with our findings that the human synovial sTCR-γδ tetramer staining was not affected by the presence or absence of CD1 or MICA/B molecules. The findings in this study were made using primary cells and tumor cell lines. Future studies will attempt to extend these results to analyses of sTCR-γδ tetramer histologic staining of primary tissues as well as tumors and inflamed synovium to determine the spectrum of TCR-γδ ligand expression at these sites. Screening primary tumors for binding of sTCR-γδ tetramer may also help identify tumors that may benefit from immunotherapy with Vδ1 γδ T cells. In addition, identifying the ligands in inflamed synovium or intestinal epithelium will provide therapeutic strategies for manipulating the function of infiltrating γδ T cells. Disclosures The authors have no financial conflicts of interest. Acknowledgments We thank Dr. Roxana del Rio-Guerra for technical assistance with flow cytometry, as well as the Harry Hood Bassett Flow Cytometry and Cell Sorting Facility at The University of Vermont Larner College of Medicine. We thank Drs. Mark Davis and Naresha Saligrama for providing the human soluble TCR-αβ. We also thank the Vermont Genetics Network National Institutes of Health IDeA Networks of Biomedical Research Excellence program and the Vermont Center for Immunology and Infectious Diseases National Institutes of Health Centers of Biomedical Research Excellence program for support of the mass spectrometry facility. Footnotes This work was supported by National Institutes of Health Grants AI107298, GM118228, and AI119979 (to R.C.B.), 8P20GM103449 (to B.A.B.), HL107152 (to K.B.), and by Wellcome Trust Grants 098274/Z/12/Z (to S.D.) and 206194 (to G.J.W.). The online version of this article contains supplemental material. Abbreviations used in this article: 2-DG 2-deoxyglucose ER endoplasmic reticulum GAG glycosaminoglycan sTCR-γδ soluble TCR-γδ Treg T regulatory cell. Received April 17, 2019. Accepted August 26, 2019. Copyright © 2019 The Authors This article is distributed under the terms of the CC BY 4.0 Unported license. References ↵Born, W., C. Cady, J. Jones-Carson, A. Mukasa, M. Lahn, R. O’Brien. 1999. Immunoregulatory functions of gamma delta T cells. Adv. Immunol. 71: 77–144.OpenUrlPubMed ↵Shi, C., B. Sahay, J. Q. Russell, K. A. Fortner, N. Hardin, T. J. Sellati, R. C. Budd. 2011. Reduced immune response to Borrelia burgdorferi in the absence of γδ T cells. Infect. Immun. 79: 3940–3946. Hiromatsu, K., Y. Yoshikai, G. Matsuzaki, S. Ohga, K. Muramori, K. Matsumoto, J. A. Bluestone, K. Nomoto. 1992. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 175: 49–56. Rosat, J. P., H. R. MacDonald, J. A. Louis. 1993. A role for gamma delta + T cells during experimental infection of mice with Leishmania major. J. Immunol. 150: 550–555.OpenUrlAbstract Kaufmann, S. H., C. H. Ladel. 1994. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology 191: 509–519.OpenUrlCrossRefPubMed Tsuji, M., P. Mombaerts, L. Lefrancois, R. S. Nussenzweig, F. Zavala, S. Tonegawa. 1994. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc. Natl. Acad. Sci. USA 91: 345–349. ↵Mixter, P. F., V. Camerini, B. J. Stone, V. L. Miller, M. Kronenberg. 1994. Mouse T lymphocytes that express a gamma delta T-cell antigen receptor contribute to resistance to Salmonella infection in vivo. Infect. Immun. 62: 4618–4621. Brennan, F. M., M. Londei, A. M. Jackson, T. Hercend, M. B. Brenner, R. N. Maini, M. Feldmann. 1988. T cells expressing gamma delta chain receptors in rheumatoid arthritis. J. Autoimmun. 1: 319–326.OpenUrlCrossRefPubMed ↵Vincent, M. S., K. Roessner, D. Lynch, D. Wilson, S. M. Cooper, J. Tschopp, L. H. Sigal, R. C. Budd. 1996. Apoptosis of fashigh CD4+ synovial T cells by Borrelia-reactive fas-ligand(high) gamma delta T cells in lyme arthritis. J. Exp. Med. 184: 2109–2117. ↵Rust, C., Y. Kooy, S. Pena, M. L. Mearin, P. Kluin, F. Koning. 1992. Phenotypical and functional characterization of small intestinal TcR gamma delta + T cells in coeliac disease. Scand. J. Immunol. 35: 459–468.OpenUrlCrossRefPubMed Balbi, B., D. R. Moller, M. Kirby, K. J. Holroyd, R. G. Crystal. 1990. Increased numbers of T lymphocytes with gamma delta-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J. Clin. Invest. 85: 1353–1361.OpenUrlCrossRefPubMed ↵Peterman, G. M., C. Spencer, A. I. Sperling, J. A. Bluestone. 1993. Role of gamma delta T cells in murine collagen-induced arthritis. J. Immunol. 151: 6546–6558.OpenUrlAbstract Pelegrí, C., P. Kühnlein, E. Buchner, C. B. Schmidt, A. Franch, M. Castell, T. Hünig, F. Emmrich, R. W. Kinne. 1996. Depletion of gamma/delta T cells does not prevent or ameliorate, but rather aggravates, rat adjuvant arthritis. Arthritis Rheum. 39: 204–215.OpenUrlCrossRefPubMed Peng, S. L., M. P. Madaio, A. C. Hayday, J. Craft. 1996. Propagation and regulation of systemic autoimmunity by gamma delta T cells. J. Immunol. 157: 5689–5698.OpenUrlAbstract ↵Mukasa, A., K. Hiromatsu, G. Matsuzaki, R. O’Brien, W. Born, K. Nomoto. 1995. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J. Immunol. 155: 2047–2056.OpenUrlAbstract Girardi, M., D. E. Oppenheim, C. R. Steele, J. M. Lewis, E. Glusac, R. Filler, P. Hobby, B. Sutton, R. E. Tigelaar, A. C. Hayday. 2001. Regulation of cutaneous malignancy by gammadelta T cells. Science 294: 605–609. ↵Costa, G., S. Loizon, M. Guenot, I. Mocan, F. Halary, G. de Saint-Basile, V. Pitard, J. Déchanet-Merville, J. F. Moreau, M. Troye-Blomberg, et al. 2011. Control of Plasmodium falciparum erythrocytic cycle: γδ T cells target the red blood cell-invasive merozoites. Blood 118: 6952–6962. ↵Gentles, A. J., A. M. Newman, C. L. Liu, S. V. Bratman, W. Feng, D. Kim, V. S. Nair, Y. Xu, A. Khuong, C. D. Hoang, et al. 2015. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21: 938–945.OpenUrlCrossRefPubMed ↵Wilhelm, M., V. Kunzmann, S. Eckstein, P. Reimer, F. Weissinger, T. Ruediger, H. P. Tony. 2003. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 102: 200–206. ↵Zeng, X., Y.-L. Wei, J. Huang, E. W. Newell, H. Yu, B. A. Kidd, M. S. Kuhns, R. W. Waters, M. M. Davis, C. T. Weaver, Y. Chien. 2012. Gamma delta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 37: 524–534.OpenUrlCrossRefPubMed ↵Nielsen, M. M., D. A. Witherden, W. L. Havran. 2017. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 17: 733–745.OpenUrlCrossRef ↵Hirsh, M. I., W. G. Junger. 2008. Roles of heat shock proteins and gamma delta T cells in inflammation. Am. J. Respir. Cell Mol. Biol. 39: 509–513.OpenUrlCrossRefPubMed ↵Morita, C. T., E. M. Beckman, J. F. Bukowski, Y. Tanaka, H. Band, B. R. Bloom, D. E. Golan, M. B. Brenner. 1995. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity 3: 495–507.OpenUrlCrossRefPubMed Tanaka, Y., C. T. Morita, E. Nieves, M. B. Brenner, B. R. Bloom. 1995. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 375: 155–158.OpenUrlCrossRefPubMed ↵Bukowski, J. F., C. T. Morita, M. B. Brenner. 1999. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity 11: 57–65.OpenUrlCrossRefPubMed ↵Witherden, D. A., K. Ramirez, W. L. Havran. 2014. Multiple receptor-ligand interactions direct tissue-resident γδ T cell activation. Front. Immunol. 5: 602.OpenUrlCrossRefPubMed ↵Adams, E. J., Y. H. Chien, K. C. Garcia. 2005. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science 308: 227–231. ↵Chien, Y. H., Y. Konigshofer. 2007. Antigen recognition by gammadelta T cells. Immunol. Rev. 215: 46–58.OpenUrlCrossRefPubMed ↵Willcox, C. R., V. Pitard, S. Netzer, L. Couzi, M. Salim, T. Silberzahn, J.-F. Moreau, A. C. Hayday, B. E. Willcox, J. Déchanet-Merville. 2012. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 13: 872–879.OpenUrlCrossRefPubMed ↵Luoma, A. M., C. D. Castro, T. Mayassi, L. A. Bembinster, L. Bai, D. Picard, B. Anderson, L. Scharf, J. E. Kung, L. V. Sibener, et al. 2013. Crystal structure of V1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human T cells. Immunity 39: 1032–1042.OpenUrlCrossRefPubMed ↵Vincent, M. S., K. Roessner, T. Sellati, C. D. Huston, L. H. Sigal, S. M. Behar, J. D. Radolf, R. C. Budd. 1998. Lyme arthritis synovial gamma delta T cells respond to Borrelia burgdorferi lipoproteins and lipidated hexapeptides. J. Immunol. 161: 5762–5771. ↵Kappler, J., J. White, H. Kozono, J. Clements, P. Marrack. 1994. Binding of a soluble alpha beta T-cell receptor to superantigen/major histocompatibility complex ligands. Proc. Natl. Acad. Sci. USA 91: 8462–8466. ↵Aydintug, M. K., C. L. Roark, X. Yin, J. M. Wands, W. K. Born, R. L. O’Brien. 2004. Detection of cell surface ligands for the gamma delta TCR using soluble TCRs. J. Immunol. 172: 4167–4175. ↵Esko, J. D., T. E. Stewart, W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82: 3197–3201. ↵‘t Hoen, P. A., Y. Ariyurek, H. H. Thygesen, E. Vreugdenhil, R. H. Vossen, R. X. de Menezes, J. M. Boer, G. J. van Ommen, J. T. den Dunnen. 2008. Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucleic Acids Res. 36: e141.OpenUrlCrossRefPubMed ↵Bentley, D. R., S. Balasubramanian, H. P. Swerdlow, G. P. Smith, J. Milton, C. G. Brown, K. P. Hall, D. J. Evers, C. L. Barnes, H. R. Bignell, et al. 2008. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456: 53–59.OpenUrlCrossRefPubMed ↵Hubbard, T. J., B. L. Aken, S. Ayling, B. Ballester, K. Beal, E. Bragin, S. Brent, Y. Chen, P. Clapham, L. Clarke, et al. 2009. Ensembl 2009. Nucleic Acids Res. 37: D690–D697.OpenUrlCrossRefPubMed ↵Ballif, B. A., Z. Cao, D. Schwartz, K. L. Carraway III., S. P. Gygi. 2006. Identification of 14-3-3epsilon substrates from embryonic murine brain. J. Proteome Res. 5: 2372–2379.OpenUrlCrossRefPubMed ↵Marlin, R., A. Pappalardo, H. Kaminski, C. R. Willcox, V. Pitard, S. Netzer, C. Khairallah, A. M. Lomenech, C. Harly, M. Bonneville, et al. 2017. Sensing of cell stress by human gammadelta TCR-dependent recognition of annexin A2. Proc. Natl. Acad. Sci. USA 114: 3163–3168. Born, W., L. Hall, A. Dallas, J. Boymel, T. Shinnick, D. Young, P. Brennan, R. O’Brien. 1990. Recognition of a peptide antigen by heat shock–reactive gamma delta T lymphocytes. Science 249: 67–69. ↵Chen, H., X. He, Z. Wang, D. Wu, H. Zhang, C. Xu, H. He, L. Cui, D. Ba, W. He. 2008. Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J. Biol. Chem. 283: 12528–12537. ↵Galgani, M., V. De Rosa, A. La Cava, G. Matarese. 2016. Role of metabolism in the immunobiology of regulatory T cells. J. Immunol. 197: 2567–2575. ↵Kamiński, M. M., S. W. Sauer, M. Kamiński, S. Opp, T. Ruppert, P. Grigaravičius, P. Grudnik, H. J. Gröne, P. H. Krammer, K. Gülow. 2012. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep. 2: 1300–1315.OpenUrlCrossRefPubMed ↵Everts, B., E. Amiel, S. C. Huang, A. M. Smith, C. H. Chang, W. Y. Lam, V. Redmann, T. C. Freitas, J. Blagih, G. J. van der Windt, et al. 2014. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 15: 323–332.OpenUrlCrossRefPubMed ↵Vander Heiden, M. G., L. C. Cantley, C. B. Thompson. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033. ↵Li, Z., Y. Jiao, Y. Hu, L. Cui, D. Chen, H. Wu, J. Zhang, W. He. 2015. Distortion of memory Vδ2 γδ T cells contributes to immune dysfunction in chronic HIV infection. Cell. Mol. Immunol. 12: 604–614.OpenUrl ↵Li, Z., W. Li, N. Li, Y. Jiao, D. Chen, L. Cui, Y. Hu, H. Wu, W. He. 2014. γδ T cells are involved in acute HIV infection and associated with AIDS progression. PLoS One 9: e106064. ↵Firestein, G. S. 2005. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J. Clin. Rheumatol. 11(3 Suppl.): S39–S44.OpenUrlCrossRefPubMed ↵Aydintug, M. K., C. L. Roark, J. L. Chain, W. K. Born, R. L. O’Brien. 2008. Macrophages express multiple ligands for gammadelta TCRs. Mol. Immunol. 45: 3253–3263.OpenUrlCrossRefPubMed
|

 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...