Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
July 11, 2024 3:35 AM
|
Research has unveiled the intricate cellular landscape of healthy human hamstring tendons, shedding new light on this vital yet often overlooked component of our musculoskeletal system.

|
Scooped by
Gilbert C FAURE
June 8, 2024 1:56 AM
|
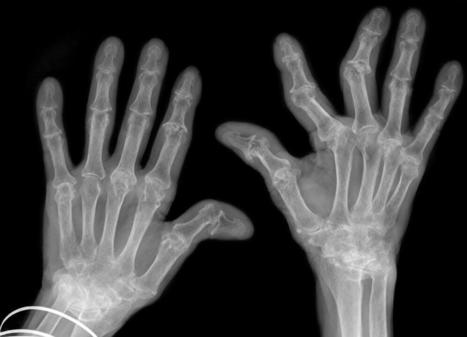
A EULAR consensus group has established classification criteria for overall hand osteoarthritis (OA) and its subtypes (interphalangeal OA and thumb base OA).The criteria require two mandatory criteria to be met:must have symptoms (pain, aching and/or stiffness) in at least one target joint on most days of the previous 6 weeks.

|
Scooped by
Gilbert C FAURE
April 24, 2024 4:44 AM
|
The Stanford Medicine researcher was known for his groundbreaking work and his generous spirit as a mentor and colleague.

|
Scooped by
Gilbert C FAURE
April 1, 2024 5:07 AM
|
<em>Arthritis Care & Research</em> is a rheumatology journal from the American College of Rheumatology and the Association of Rheumatology Health Professionals.

|
Scooped by
Gilbert C FAURE
March 15, 2024 3:52 PM
|
Les 29 entreprises de la Prosthesis Valley entre Chaumont et Nogent en Haute-Marne, un ancien territoire spécialisé dans la coutellerie, sont devenues en quelques années des leaders mondiaux sur le marché médical.

|
Scooped by
Gilbert C FAURE
February 29, 2024 2:11 AM
|
Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects millions of individuals worldwide, causing pain, inflammation, and joint damage. Despite its prevalence, RA can be misunderstood, leading to challenges in diagnosis and management.

|
Scooped by
Gilbert C FAURE
February 24, 2024 8:09 AM
|
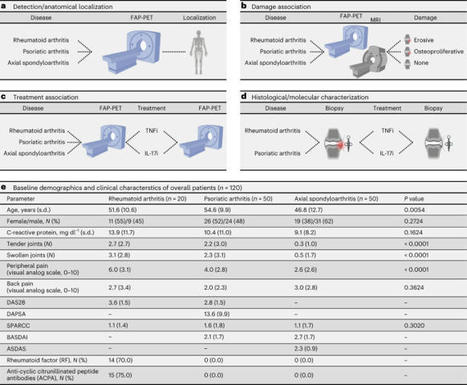
Here the authors use positron emission tomography to visualize fibroblasts in patients with arthritis and combined with spatial transcriptomic data show that these cells undergo a phenotypic shift upon resolution of inflammation.

|
Scooped by
Gilbert C FAURE
February 19, 2024 3:22 AM
|
Si la revue #Rhumatos fête ses 20 ans, la #rhumatologie a connu des bouleversements significatifs pendant cette même période comme en témoigne ce numéro…

|
Scooped by
Gilbert C FAURE
February 13, 2024 11:17 AM
|
SNDS et maladie rare : le syndrome de Sjögren (SS) en France (#Publication)
🎯 Objectifs : Estimer la prévalence, l'incidence, la mortalité et les coûts de…

|
Scooped by
Gilbert C FAURE
February 1, 2024 1:45 PM
|

|
Scooped by
Gilbert C FAURE
January 22, 2024 6:18 AM
|
EULAR has published the 2023 updated recommendations for the optimal non-pharmacological management of hip and knee osteoarthritis (OA). Non-pharmacological treatments for OA are as important and impactful at improving pain, function and quality of life in OA. These EULAR recommendations were originally published in 2013. This update began in 2022 with a multidisciplinary Task Force (25 members from 14 European countries) who conducted a systematic literature review that became the basis for updated recommendations.

|
Scooped by
Gilbert C FAURE
January 10, 2024 11:01 AM
|
A Swedish study examine the genetic contribution to various rheumatic/musculoskeletal diseases (RMDs) and showed that osteoarthritis (OA) had a large heritability compared with other RMDs.

|
Scooped by
Gilbert C FAURE
December 13, 2023 4:40 AM
|
Article Text Article menu PDF Viewpoint Goodbye to the term ‘ankylosing spondylitis’, hello ‘axial spondyloarthritis’: time to embrace the ASAS-defined nomenclature http://orcid.org/0000-0002-5781-158XDésirée van der Heijde1, http://orcid.org/0000-0003-2246-1986Anna Molto2,3,...
|

|
Scooped by
Gilbert C FAURE
June 10, 2024 9:08 AM
|
Exercising and stretching can help relieve lower back pain. Learn the best exercises for your lower back, where to start, and how to prevent future pain.

|
Scooped by
Gilbert C FAURE
April 30, 2024 11:26 AM
|

|
Scooped by
Gilbert C FAURE
April 7, 2024 5:02 AM
|
Breaking Barriers Through Multi-Disciplinary Osteoarthritis (OA) Research - the OARSI - Osteoarthritis Research Society International World Congress in Vienna,…

|
Scooped by
Gilbert C FAURE
March 17, 2024 6:20 AM
|
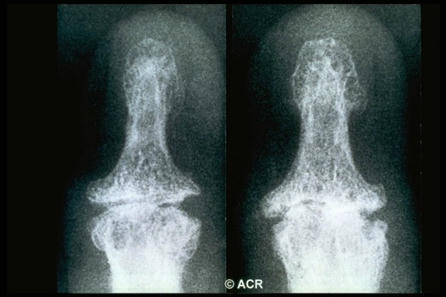
Today I want to highlight the paper of Marta Favero, Roberta Ramonda and team on erosive hand osteoarthritis.
This disease is most common in women and…

|
Scooped by
Gilbert C FAURE
March 8, 2024 7:09 AM
|
New research has identified apolipoprotein E expressed by fibroblasts and macrophages in the infrapatellar fat pad and synovium as a pathogenetic mediator and potential therapeutic target in knee osteoarthritis.

|
Scooped by
Gilbert C FAURE
February 25, 2024 1:07 PM
|
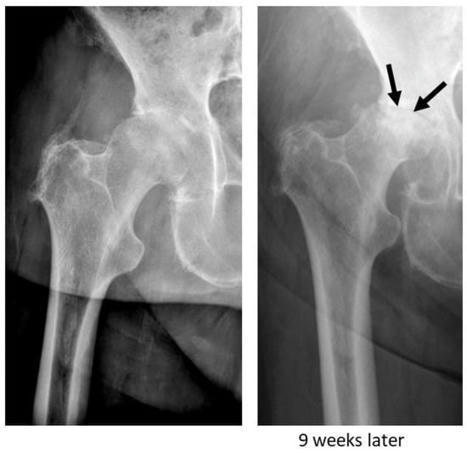
Background: Rapidly destructive osteoarthritis (RDO) of the hip joint is characterised by the rapid destruction of the femoral head with or without acetabular involvement. There has been increasing interest in this disease over the past years; however, the entity is still poorly understood, and...

|
Scooped by
Gilbert C FAURE
February 19, 2024 6:54 AM
|
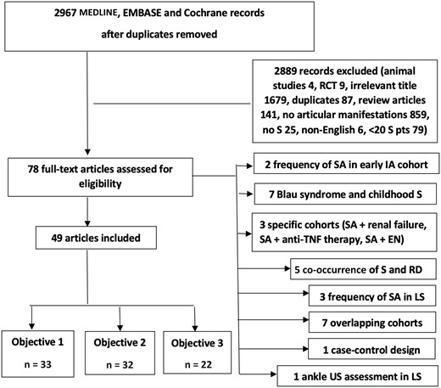
AbstractObjectives. To characterize joint involvement (JI) in sarcoidosis, a systematic search of MEDLINE, EMBASE and Cochrane Library was conducted from incept

|
Scooped by
Gilbert C FAURE
February 16, 2024 4:47 AM
|
Proud to share the results from our Nature Medicine paper that was recently published:
Ground breaking results for patients with erosive hand osteoarthritis:…

|
Scooped by
Gilbert C FAURE
February 13, 2024 11:12 AM
|
IntroductionCrystal-induced arthropathies (CiAs) are common conditions caused by the deposition of crystals within articular and periarticular tissues.1 2 The three types of crystals that are mainly involved in the pathogenesis of these diseases are monosodium urate (MSU) in gout, calcium...

|
Scooped by
Gilbert C FAURE
January 24, 2024 6:04 AM
|
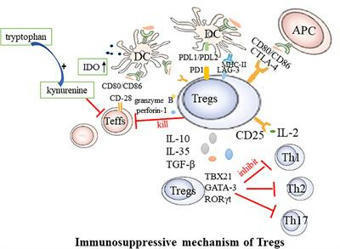
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune condition marked by inflammation of the joints, degradation of the articular cartilage, and bone resorption. Recent studies found the absolute and relative decreases in circulating regulatory T cells (Tregs) in RA patients. Tregs are a unique type of cells exhibiting immunosuppressive functions, known for expressing the Foxp3 gene. They are instrumental in maintaining immunological tolerance and preventing autoimmunity. Increasing the absolute number and/or enhancing the function of Tregs are effective strategies for treating RA. This article reviews the studies on the mechanisms and targeted therapies related to Tregs in RA, with a view to provide better ideas for the treatment of RA.

|
Scooped by
Gilbert C FAURE
January 15, 2024 10:03 AM
|
In the burgeoning domain of orthopedic therapeutic research, Platelet-Rich Plasma (PRP) has firmly established its position, transforming paradigms ranging from tissue regeneration to the management of chondral lesions.

|
Scooped by
Gilbert C FAURE
December 20, 2023 8:37 AM
|
WHAT IS ALREADY KNOWN ON THIS TOPICMonoclonal therapy for one inflammatory disease may paradoxically trigger another inflammatory disease.Recent case reports have implicated an association between anti-IL-5 (IL, interleukin) antibody therapy used to treat severe asthma and the development of rheumatoid arthritis (RA).WHAT THIS STUDY ADDSOut of 142 patients within our asthma service taking anti-IL-5 antibody therapy for at least 1 month, and with a mean duration of 3.5 years on therapy, only one developed RA suggesting that RA is a relatively uncommon complication in the short-medium term.HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICYTreating clinicians should be mindful of the possibility of developing inflammatory arthritis following the initiation of anti-IL-5 therapy and ensure appropriate review and assessment should their patient develop arthralgia as, while uncommon, these could represent a significant source of morbidity.IntroductionThere has been a wide adoption of monoclonal antibody therapy in rheumatology, respiratory medicine, and an increasing number of specialties for the treatment of many inflammatory diseases. Of particular interest is that monoclonal therapy for one inflammatory disease may paradoxically trigger another inflammatory disease. Pertinent examples include tumour necrosis factor inhibitor therapy triggering multiple sclerosis,1 interleukin 17 (IL-17) therapy for psoriasis linked to inflammatory bowel disease2 and more recently IL-4/13 blockade used for atopic dermatitis being associated with de novo psoriasis and arthritis.3 4Arthralgias are a known adverse effect of anti-IL-5 biologics,5 6 however, a few recent case reports have found this association may extend to inflammatory arthritis such as RA.7 8 The prevalence of these findings across a wider cohort of patients remains relatively unknown. Here we present an audit from a large, single-centre’s severe asthma service which looks for the prevalence of RA across all patients being treated with mepolizumab and benralizumab, two commonly used anti-IL-5 therapies.MethodsAll patients with severe eosinophilic asthma across the Leeds Teaching Hospitals NHS Trust’s (LTHT) Respiratory Service, who had received at least 1 month of mepolizumab or benralizumab therapy, were included in this clinical audit.Each patient’s electronic records, including hospital records, clinic letters, general practitioner (GP) records and electronic pathology results were searched. We recorded whether patients had presented with any signs or symptoms of synovitis (eg, joint pain, swelling and tenderness) either prior- or post-commencing biologics, whether their serology (rheumatoid factor (RF) and/or anti-CCP antibody (ACPA)) and acute phase reactants (C- Reactive Protein (CRP) and/or Erythrocyte Sedimentation Rate (ESR)) had been measured and the timing and duration of their symptoms. Using this information, we then calculated the number of points each patient with symptoms would score on the ACR/EULAR 2010 Rheumatoid Arthritis classification criteria.9 We also recorded the dose of routine steroids the patients were receiving prior to starting biologics, and whether they were weaned off steroids within 1 year of commencing biologics. Finally, we recorded whether the patients had been seen in our early arthritis clinic and received a formal diagnosis on an inflammatory arthritis.ResultsA total of 142 patients (57 males, 85 females and mean age 58.2 years old) were being treated with anti-IL-5 biologics under the LTHT’s severe asthma clinic, with a mean duration of 3.5 years on therapy. Eighty-nine were on mepolizumab and 53 on benralizumab. The mean daily dose of steroids prior to starting anti-IL-5 therapy was 6.0 mg prednisolone, reducing to 3.1 mg at 1 year post-therapy. Seventy-five patients were steroid-free after 1 year of therapy.Only one patient among 500 patient years of exposure to anti-IL-5 therapy received a formal diagnosis of RA suggesting an overall annual incidence of 20 cases per 10 000 patients (95% CI 2.8 to 142). This man in his 70s presented to our early arthritis clinic 18 months after having been started on mepolizumab for his severe eosinophilic asthma. Prior to starting biologics, his asthma had been poorly controlled with salbutamol, budesonide/formoterol combination inhaler, tiotropium inhalers and daily low-dose oral corticosteroids. Within weeks, he developed symmetrical arthralgia involving small joints, particularly his wrists and knuckles, as well as significant early morning stiffness lasting more than 1 hour. On examination, he had clinical synovitis in the wrists and metacarpophalangeal joints bilaterally, as well as right shoulder capsulitis with limiting range of motion.Blood tests revealed a raised CRP of 24 mg/L, White Cell Count (WCC) 8.77 10 × 9 /L, RF of 263.2 iu/mL (normal<14.0) and an ACPA of >300 U/mL (normal<2.99). Ultrasound imaging of the hands and wrists showed bilateral grade II grey scale with grade II power Doppler (figure 1) with bilateral wrist erosions. There was hypoechogenicity of the left extensor carpi ulnaris tendon with some associated grey scale and power Doppler. MRI of the left hand revealed extensive subchondral bone marrow oedema (figure 1) and multiple erosions across all carpal bones and carpometacarpal joints (figure 1).<img width="342" alt="Figure 1" height="440" class="highwire-fragment fragment-image" src="https://rmdopen.bmj.com/content/rmdopen/9/4/e003583/F1.medium.gif">Download figure Open in new tab Download powerpoint Figure 1 (A) Fat suppression MRI of the left wrist and MCPs showing extensive bone oedema (white arrows) and joint effusion (black asterisk). (B) T1-weighted MRI of the left wrist showing diffuse erosions of the left wrist (white arrows). (C) Longitudinal ultrasound image showing synovitis of the right wrist with grey scale (white asterisk) and power Doppler (white arrow).He was diagnosed with RA as per the American College of Rheumatology (ACR)/EULAR classification criteria and started on prednisolone 10 mg daily to control the inflammation, followed by sulfasalazine 1 month later as the disease modifying agent. He was followed-up in rheumatology clinic 2 months later and showed significant improvements: the joint pain and swelling had settled, and while he still experienced early morning stiffness, this was less debilitating. His inflammatory markers had also resolved with CRP<5.0 mg/L and WCC 9.31 10 × 9 /L.Of the remaining 141 patients, 16 developed bilateral polyarthralgia of greater than 1 month duration (eight mepolizumab and eight benralizumab), with a median onset of 12 months after commencing a biological therapy. Of these patients 9/16 were tested for RF and ACPA and in all cases, their serology was negative; 15/16 patients had acute phase inflammatory markers measured and these were only elevated in three patients. All 16 of these patients were on a maintenance dose of prednisolone prior to starting the biologic (mean dose 10.1 mg/day), with 10 of them completely weaned off steroids within 12 months.Using the information available from the patient’s electronic records, the mean number of points scored on the ACR/EULAR RA criteria was 3.2 (range 1–6). The patient who scored six points was reviewed in the early arthritis clinic and the symptoms were felt to be more in keeping with osteoarthritis than an inflammatory arthritis. Similarly, none of the other patients had received a confirmed diagnosis of inflammatory arthritis by either their GP or by a rheumatologist.Only one other patient became newly RF positive (17.1 iu/mL), 1 month after commencing mepolizumab; however, this seemed to be an incidental finding as the patient had a broad set of bloods taken while admitted to the intensive care unit for a severe exacerbation of asthma, and at no point since has complained of rheumatological symptoms.We were unable to access the GP records for 37 patients and as such could not review whether they had presented to their GPs with new rheumatological symptoms. However, we were able to access their pathology test records electronically and found no evidence of positive RA serology in any of these patients and no rheumatological referrals to our centre that has a well-developed early RA network.DiscussionThere is an emerging interest in IL-5 blockade and the potential development of RA. We present a single-centre’s experience of 500 patient years on anti-IL-5 monoclonal antibody exposure therapy for severe asthma.As expected, arthralgias were a relatively common side-effect of anti-IL-5 therapy. As for progression to RA, we found only one convincing case. While relatively low, the implied annual incidence of 20 cases per 10 000 patients is several fold higher than the annual incidence of RA in the UK (1.5 per 10 000 men and 3.6 per 10 000 women).10 Given the wide CIs, however, no firm conclusions can be offered in relationship to our single case and to the relative risk of RA following anti-IL-5 therapy.A major confounding variable is the weaning of steroids in most patients started on biologics. This poses a challenge in associating the development of symptoms with the initiation of the anti-IL-5 therapy, as opposed to the withdrawal of steroids unmasking a pre-existing disease. Additionally, one must consider whether the risk of developing RA is modified by the underlying condition, and indeed there is some evidence to suggested that asthma may be positively associated with RA.11 However, these population-based studies look at asthma as a whole, rather than divided into its endotypes (eg, eosinophilic vs neutrophilic asthma) and as such these have not yet challenged the conventional belief that Th1 and Th2 diseases are inversely related.Emerging evidence has implicated a core role for regulatory eosinophils (rEos) in the resolution of RA.12 In murine models of RA, the expansion of rEos in the synovial fluid as a by-product of inducing eosinophilic asthma was sufficient in bringing about remission of arthritis, and inhibiting the IL-5 pathway would subsequently induce relapse of the arthritis.12 Further evidence supporting a role for rEos in RA can be found at a genetic level where Eotaxin-3, one of the main drivers of eosinophil recruitment, has single nucleotide polymorphisms associated with RA13 and from studying the role of IL-5 in Th2 responses to Helminth infections,14 with mouse models of RA also identifying Helminth infections as protective.15 Hence, the suggestion that the expansion of eosinophils in the synovium ‘regulate’ the proinflammatory Th1 pathways driving synovial inflammation.12 This invites the notion that in a patient with subclinical, yet endogenously controlled, synovial inflammation, removing rEos by administering anti-IL-5 therapeutics may tip the balance in favour of inflammation and permit symptomatic disease. However, if there is little proinflammatory Th1 synovial activity in the first place, then inhibiting rEos with anti-IL-5 biologics may be insufficient to precipitate an inflammatory arthritis.Interestingly, there is debate as to whether rEos are depleted to varying degrees depending on the anti-IL-5 biologic used. In mice, inflammatory eosinophils (iEos)—the primary targets of anti-IL-5 biologics in asthma—may be dependent on IL-5 for activity, whereas rEos may not be.16 This would suggest that benralizumab, a high-affinity IL-5 receptor antagonist,17 would deplete both iEos and rEos through NK-mediated killing, whereas mepolizumab, an anti-IL-5 monoclonal antibody,17 may deplete iEos but keep rEos intact. However, this idea has recently been challenged with evidence that anti-IL-5 treatment depletes all populations of eosinophils.18 Whether this distinction would result in a different pattern of adverse effects in patients remains unclear, notably as the patient who developed RA in this report was receiving mepolizumab.As an audit, this study serves to identify the prevalence of a relatively rare complication of anti-IL-5 therapy. We were unable to find clear evidence for a pattern of emergent RA nor other inflammatory arthritis in our cohort of 142 patients. Further studies may be required to characterise the nature and significance of these findings in clinical groups and to identify whether there is an actual association between novel anti-IL-5 biologics and RA.Data availability statementThe data that support the findings of this study are available upon reasonable request.Ethics statementsPatient consent for publicationConsent obtained directly from patient(s).Ethics approvalThis study was registered as a clinical audit and given the retrospective nature of the data collection process did not require formal ethical approval. In completing this audit, full ethical standards were upheld in accordance with the principles of clinical governance. From the one patient whose details were discussed in more detail we have gained full written consent.References↵Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 2001;57:1885–8. doi:10.1212/wnl.57.10.1885OpenUrlCrossRefPubMed↵Hohenberger M, Cardwell LA, Oussedik E, et al. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat 2018;29:13–8. doi:10.1080/09546634.2017.1329511OpenUrlPubMed↵Bridgewood C, Newton D, Bragazzi N, et al. Unexpected connections of the IL-23/IL-17 and IL-4/IL-13 cytokine axes in inflammatory arthritis and enthesitis. Semin Immunol 2021;58:101520. doi:10.1016/j.smim.2021.101520OpenUrl↵Bridgewood C, Wittmann M, Macleod T, et al. T helper 2 IL-4/IL-13 dual blockade with dupilumab is linked to some emergent T helper 17‒Type diseases, including seronegative arthritis and enthesitis/enthesopathy, but not to humoral autoimmune diseases. J Invest Dermatol 2022;142:2660–7. doi:10.1016/j.jid.2022.03.013OpenUrl↵Harrison T, Canonica GW, Chupp G, et al. Real-world Mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J 2020;56:2000151. doi:10.1183/13993003.00151-2020↵Liu W, Ma X, Zhou W. Adverse events of benralizumab in moderate to severe eosinophilic asthma: a meta-analysis. Medicine (Baltimore) 2019;98:e15868. doi:10.1097/MD.0000000000015868↵Kawabata H, Satoh M, Yatera K. Development of rheumatoid arthritis during anti-Interleukin-5 therapy in a patient with refractory chronic eosinophilic pneumonia. J Asthma Allergy 2021;14:1425–30. doi:10.2147/JAA.S342993OpenUrl↵Dupin C, Morer L, Phillips Houlbracq M, et al. Arthritis, a new adverse effect of anti-Il5 Biologics in severe asthma patients. European Respiratory Journal 2022;60:2432. doi:10.1183/13993003.congress-2022.2432OpenUrlCrossRef↵Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American college of rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. doi:10.1002/art.27584OpenUrlCrossRefPubMedWeb of Science↵NICE guideline. Overview: rheumatoid arthritis in adults: management [Guidance, NICE]. 2018. Available: https://www.nice.org.uk/guidance/ng100 [Accessed 25 Sep 2023].↵Rolfes MC, Juhn YJ, Wi C-I, et al. Asthma and the risk of rheumatoid arthritis: an insight into the heterogeneity and phenotypes of asthma. Tuberc Respir Dis (Seoul) 2017;80:113–35. doi:10.4046/trd.2017.80.2.113OpenUrl↵Andreev D, Liu M, Kachler K, et al. Regulatory eosinophils induce the resolution of experimental arthritis and appear in remission state of human rheumatoid arthritis. Ann Rheum Dis 2021;80:451–68. doi:10.1136/annrheumdis-2020-218902OpenUrlAbstract/FREE Full Text↵Guellec D, Milin M, Cornec D, et al. Eosinophilia predicts poor clinical outcomes in recent-onset arthritis: results from the ESPOIR cohort. RMD Open 2015;1:e000070. doi:10.1136/rmdopen-2015-000070↵Mishra PK, Palma M, Bleich D, et al. Systemic impact of intestinal helminth infections. Mucosal Immunol 2014;7:753–62. doi:10.1038/mi.2014.23OpenUrlCrossRefPubMed↵Osada Y, Shimizu S, Kumagai T, et al. Schistosoma Mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol 2009;39:457–64. doi:10.1016/j.ijpara.2008.08.007OpenUrlCrossRefPubMed↵Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 2016;126:3279–95. doi:10.1172/JCI85664OpenUrlCrossRefPubMed↵Caminati M, Menzella F, Guidolin L, et al. Targeting eosinophils: severe asthma and beyond. Drugs Context 2019;8:212587. doi:10.7573/dic.212587OpenUrl↵Dolitzky A, Grisaru-Tal S, Avlas S, et al. Mouse resident lung eosinophils are dependent on IL-5. Allergy 2022;77:2822–5. doi:10.1111/all.15362OpenUrl
|

 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...