Your new post is loading...
 Your new post is loading...

|
Scooped by
Rémy TESTON
April 18, 2017 3:36 AM
|
PARIS (TICpharma) - Roche Diabetes Care France (groupe Roche) prépare l'arrivée dans l'Hexagone du premier capteur implantable de mesure du glucose en continu (CGM), Eversense, pour les diabétiques traités par insuline, et annonce un essai clinique français pour septembre.
Operating extensively within the digital healthcare industry, both as a startup, as well as a digital marketing agency supporting global pharmaceutical companies has taught us that both have a need that the other can readily offer. As pharma and consumer healthcare companies are quickly realising, the future of healthcare lies in the technologies of tomorrow. For digital healthcare startups on the other hand, the opportunities to see their dreams realised have never been more exciting. So, how and why can both the pharma company and the startup make positive headway here.
Via Giuseppe Fattori
Social media has been a big focus for pharma marketers for a while now. By my count, at least 30-45% of ePharma’s agenda from the 2014 NY conference was focused on the subject, and there is a whole cottage industry of other conferences specifically for social media fin the pharma industry. If you spend any time following pharma folks on Twitter, you can find tons of tweets on the subject and create whole feeds for hashtags like #socpharm, #hcsm, #pharmsm, etc. I say it’s time to move on. You read correctly. Before some of you go indiscriminately crazy and lambaste me in the comments for the mere suggestions that social isn’t important, let me offer some points of clarification. As it relates to corporate communications, I think using social media is a no brainer. For J&J, Pfizer, AZ, et. al., using social channels effectively is essential for reputation management, stockholder news, crisis management, etc. It’s the cost of doing business in the digital world we live in. Additionally, using social platforms to seed content is just fine, as long as you’re not expecting huge results. I’m a firm believer in a distributed content strategy, but 99% of the time, pharm brands place content in social platforms with the comments sections (or anything else even remotely ‘social’) disabled. I believe the whole use of the medium needs to be seriously rethought. Simply put, there are serious challenges for using (and I mean really using) social media for a pharma brand. For instance: Fostering dialogue and conversations isn’t the business that pharma brands are inThe marketing teams assigned to those brands aren’t built to sustain the kinds of relationships necessary to succeedPR and marketing rarely coordinate within a given brandThe regulatory organizations (FDA or otherwise) will only let you discuss what’s exactly in the product’s label, andUsers, by all indications, aren’t interested in pharma infringing on their timelines and feeds Defining social media
The term “social media” has been hijacked by the pharma industry, and thus, needs to be properly re-defined in order to better comprehend my argument. Social media, as defined by Wikipedia, is “…interaction among people in which they create, share, and/or exchange information and ideas in virtual communities and networks.” If you read this carefully, you begin to understand my point. Pharma does almost none of these things. While the creation of content is part and parcel to the pharma marketing regimen, I would argue that the minute your regulatory team requires you shut off sharing or comments features, the social media aspects of your programs cease to exist. If social media is about the collaboration of ideas and the sharing of communication, is it really a social program any more if the direction of those communications is entirely one-way? Hey hey, ho ho. The FDA is slow slow slow
In 2009 the FDA held hearings to gather input from industry about what it should consider when eventually releasing guidance on social media marketing. From that moment, I’ve heard the FDA being used as a prop for why brands couldn’t or shouldn’t engage in social channels. The reasoning was that brands wanted to clearly understand what the guidance would be, or the fear of launching something that would eventually be deemed non-compliant thereby incurring a warning letter. I think those and the myriad of other excuses assigned to the FDA were false-flags hiding a deeper issue. If the business case for social utilization were solid, and most would have you believe that it is, then clarification from FDA should open the flood gates for social media programs. But social media just isn’t really understood by brand marketers, and more importantly, the more one examines the business case for it, the less sense it actually makes for pharma products. Don’t believe me? I can prove it. The second week of January 2014, the FDA released draft “Guidance for Industry Fulfilling Regulatory Requirements for Postmarketing Submissions of Interactive Promotional Media for Prescription Human and Animal Drugs and Biologics.” Despite being woefully behind schedule in releasing the full social media guidance it promised, this was at least something from the FDA that clarified its position in some small part. Most notable of the guidance was that FDA finally provided clarity about one of the aspects of social media that the industry has been clamoring for the most: working with bloggers and content creators to disseminate branded information. You would think, given that it’s been more than 90 days later, we’d have seen movement towards these kinds of programs. After all, we were told guidance, any guidance, was necessary so brands could finally move forward with social media without running afoul of the FDA. As of this posting, there has been nothing even remotely “social” being done. As part of running the Social Media Wiki, I have a number of bots and alerts to let me know when a whole host of pharma products get discussed online. I may be slow to output updates to the wiki, but the data coming in is timely. It is possible that I missed something, but I doubt it. It’s time we had “the talk”
I hate to break the news, but social communications is not the business that pharma is in. If every reply, response, or retweet has to take 24 hours (or more) to run through a legal team before going live, I think we’ve gone well beyond any reasonable concept of conversation or dialogue. I think it’s also reasonable to assume if a patient has a question about a pharma product they don’t want to wait 24-72 hours for a response. But even if the lag time were lesser, would it even matter? When you actually ask patients if they find pharma’s participation in social to be effective, the answer is a resounding “no.” According to a Deloitte study in 2012, despite almost 65% of respondents saying that they use the internet for health information, only 5% said that social networking sites from pharma were trustworthy and credible. 5%. Even if pharma were able to engage in anything close to real time, patients just simply won’t believe the information being provided. Perhaps even more damaging, according to Manhattan Research (ePharma consumer 2013) when asked how they’d like to engage with a pharma brand, only 10% indicated they’d watch a YouTube video and 11% said they’d ‘Like’ a Facebook page. (Other channels, like Twitter ranked even lower) Given those abysmal numbers, should pharma even bother? Not every brand can grow up to be Zappos. That’s ok. There are plenty of non-pharma resources out there for patients that provide beneficial real-time interactions. In all likelihood, patients will get the info they need from message boards, online resources from their insurance provider, or increasingly, their physician via email or text. The real-time interactions that would benefit patients are more closely aligned with the roles played by the physician, pharmacists or insurance providers are playing online. Those industries are getting better and better at using social channels to help patients while pharma falls further and further behind. So instead, perhaps it’s time to more properly focus on what maximizing the value that pharma CAN provide. Software As A Service: The Real “Pill +”
At its essence, pharma provides medicines that patients utilize to combat illness. The business is one of product development, engineering, distribution, and use. I don’t care what pill, injectable, brand, or biologic you’re marketing, your #1 problem is usually compliance. Almost every pharma product except Viagra or contraception has a compliance drop off curve that plummets somewhere around the 90 day mark. (There are of course variances, but this is pretty typical). According to a report by Harris Interactive, roughly half of all prescriptions for drugs to be taken on an ongoing basis are either not completed or are never filled in the first place. The information on how to take a medication is widely available. Patients receive a package insert with their medication detailing the information about what they’ve been prescribed and how to take it. Their doctor probably spent some time walking them through what they need to know as well. For its part, pharma has been very successful educating patients about these topics. Say what you will about the brand.com for pharma (and I have), they still do the yeoman’s work of providing information to patients, detailing information about what a product does, how to take it, and what side effects it may cause. If a patient does have a question about a product, chances are the information, (or rather, the information pharma is allowed to share), is detailed rather well on its website. So if compliance is the problem, software services (like mobile tools) not social media, are the most likely solutions. Compared to the abysmal numbers for social media, according to Kantar Health study, 90% of patients would like an app or online service to help them manage their condition or track taking their medications, provided it was recommended by their physician. Pharma is very good at developing and distributing the molecules and biologics to help patients, but has an enormous opportunity to develop technologies and services in a parallel path that assist the patient when taking their medicine. At its core, the pharma industry is rooted in science and technology, developing the software and services to accentuate a product isn’t that far outside of it’s cultural wheelhouse. Perhaps, given the desire by patients for services, not social conversations, pharma should refocus its efforts on creating the technologies and tools that give patients what they need to manage their conditions, and leave the chatting to someone else. In my next post, I’ll take a look at the most promising areas for pharma to focus it’s efforts in software and services. In the mean time, drop me a comment and tell me what you think. - See more at: http://www.doseofdigital.com/2014/03/time-pharma-give-social-media-ghost/?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+DoseOfDigital+(Dose+of+Digital+-+Improving+Healthcare+Through+Digital+Technology)#sthash.6h1y1D3A.dpuf
Via Plus91, Chanfimao, Lionel Reichardt / le Pharmageek

|
Scooped by
Rémy TESTON
March 10, 2017 11:25 AM
|
Ni rachat de start-up, ni création de cellule innovation… Le deuxième groupe pharmaceutique français préfère les partenariats opérationnels. Objectif : en signer une quinzaine par an.

|
Rescooped by
Rémy TESTON
from Social Media and Healthcare
March 7, 2017 2:35 AM
|

|
Scooped by
Rémy TESTON
March 4, 2017 3:42 AM
|
Le groupe pharmaceutique français Sanofi a annoncé jeudi le lancement d'essais cliniques numériques permettant en particulier de suivre le

|
Scooped by
Rémy TESTON
February 15, 2017 3:19 AM
|
MADISON (Wisonsin) (TICpharma) - La société américaine Propeller Health a annoncé le 8 février un partenariat avec Novartis afin de développer un capteur sur mesure pour connecter l'inhalateur Breezhaler* à sa plateforme de traitement des données liées aux troubles respiratoires.

|
Rescooped by
Rémy TESTON
from Pharma Hub
February 12, 2017 3:04 AM
|
While most healthcare stakeholders are eager to embrace digital health, the pharmaceutical sector has been somewhat reluctant to join the digital health bandwagon. Some of the forward-thinking pharma companies are just now awakening to the opportunity for digital health to strengthen their businesses.
Via Philippe Marchal

|
Rescooped by
Rémy TESTON
from Digital Disruption in Pharma
February 7, 2017 1:49 PM
|
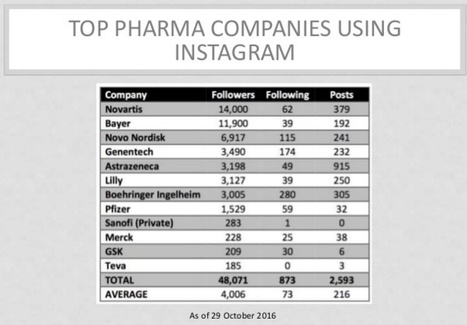
Drugmakers have claimed their Instagram turf by launching corporate pages first then moving to unbranded and branded ones. Medtronic and Novartis are actively engaging on the platform through their corporate handles, each with more than 14,000 followers. Then there are companies such as Sanofi that have created a handle, but not yet activated it with posts, says Intouch Solutions EVP Wendy Blackburn. [Read “Pharma on Instagram: How Top Drug Companies Use It Today & May Use It Tomorrow”; http://sco.lt/8lqefp] UCB global multichannel engagement solutions lead Greg Cohen says “I've noticed pharma companies have grabbed the Instagram names for their products, but there are no posts yet,” he continues. “They're probably getting them set up and holding them in case they decide to move into it.” What makes Instagram so potentially valuable to pharma and healthcare marketers is its visual story-telling capability. As Salowski notes, it is “incredibly sticky” when it comes to engagement. The average Instagram user spends 21 minutes per day in the app, and users upload more than 95 million photos per day, which garner more than 3.5 billion likes. Thus, Instagram allows companies to not just break down complicated medical issues, but also “distill them into bite-sized components, which patients can consume more easily,” Cohen explains. “Users can either learn and teach themselves or share them with their networks, because the highly visual nature makes it easy for them to share their stories.” “Instagram makes more sense for disease categories for younger people,” Blackburn explains. “Diabetes is where I see the biggest opportunity and activity, because it has always been a social disease category. It's not right for Alzheimer's or Parkinson's right now,” she adds. As with their presence on other social media platforms, drugmakers on Instagram face the challenge of regulatory compliance. This is no small hurdle, given how the FDA continues to fine-tune guidelines about safety information within limited-character formats. Instagram has a character limit of 2,200, which affords more flexibility than Twitter's 140 characters, but still may not be enough. If nothing else, Duchesnay's experience with Kardashian West reinforced the importance of hewing to the prescribed rules. Another concern drugmakers may have is how to deal with negative comments and online trolls. While companies can disable comments altogether, they're wary of limiting the back and forth with patients, physicians, and caregivers. That's why Blackburn singles out Pfizer's corporate Instagram page, thought to be one of the most skilled in moderating, deflecting, and otherwise dealing with negative comments. “Pfizer can delete the comments, but they don't,” she notes. “There are always going to be trolls and haters. Deleting them sends the wrong message, because you're there to engage. At the same time, you can't play into the game of responding to every troll. Ignore the ones that came on to bait you.” Further Reading: “Instagram Offers Pharma Marketers Better Tools Than Facebook to Block Those "Nasty" Comments”; http://sco.lt/5tW1Pl ;“Now May Be the Time for Pharma to Get Serious About Instagram”; http://sco.lt/79K5JZ ;“Should #Pharma Consider Instagram for Promotion & Patient Engagement?”; http://sco.lt/8CkdfN
Via Pharma Guy

|
Rescooped by
Rémy TESTON
from Social Media and Healthcare
January 16, 2017 4:10 PM
|
While the three granddaddy social networks, Facebook, Twitter and YouTube, have spent a decade publicly duking it out for share of human attention-span and commercial usefulness, LinkedIn has quietly and efficiently evolved to become an essential pillar of corporate practice, for individuals and organizations alike. In fact, it's almost impossible to imagine that its founders could have wholly foreseen or planned for the true magnitude of its impact on business today. LinkedIn was unleashed way back in 2003—two years ahead of YouTube, it should be noted—and attracted just 4,500 members during its first month of operation. By the time Twitter came into being and Facebook had thrown open its doors to anyone/everyone, both in 2006, LinkedIn had already launched its “public profiles” and had amassed close to five million users. These days, the network is available in 24 languages and boasts more than 9,200 full-time employees with offices in 30 cities around the world. Membership currently stands at more than 400 million people worldwide, 122 million of whom are in the US. See also: Pfizer's Susman: Customer-company relationship has changed LinkedIn has been something of an enigma and part of the reason has been the difficulty of pinpointing its core purpose. For a long time, it was seen as a professional advancement network, crudely dubbed “Facebook for jobs.” But while career advancement and recruitment is still a major function, over time LinkedIn has evolved into an effective publishing platform for professional content, which has had profound implications for corporate communications and marketing activities. Indeed, marketing solutions now represent 18% of LinkedIn revenues. A big part of LinkedIn's appeal lies in the high level of engagement of its members and the potential for precise targeting of content and talent searches. In the healthcare space, membership encompasses all stakeholder groups: pharma companies, patients, healthcare professionals, marketing agencies, hospitals, investors and, of course, employees (read: the talent pool). Of the largest pharma companies, AstraZeneca currently has around 455,000 LinkedIn followers, versus approximately 27,600 on Facebook. Similarly, Novartis has 794,000 LinkedIn followers versus 121,000 on Facebook), Johnson & Johnson has one million (versus 695,000) and Pfizer 1.1 million (versus 195,000). Each of these companies posts content regularly on LinkedIn. Conversely, Gilead Sciences publishes only job listings and counts just 106,000 LinkedIn followers, which illustrates the importance of posting useful content beyond career opportunities. Consider also the personal posts of individual pharmaceutical executives. Pfizer CEO Ian Read currently has 108,000 followers. His three most recent posts deal with topics as diverse as career tips for young professionals, treating diseases in developing countries and clarifying Pfizer's tax position regarding the Allergen merger. The “tips” post garnered almost 60,000 views and generated more than 200 comments. The number of US healthcare professionals using LinkedIn is currently estimated at more than four million. A 2015 survey by CMI/Compas Media Vitals of 2,152 physicians across all specialties showed that 33% used LinkedIn for professional purposes (versus just 8% for Facebook). The numbers vary between specialties, with 44% of dentists and 40% of dermatologists using LinkedIn professionally. The evolution of LinkedIn from a career network to a publishing platform has had a profound effect on social-media responsibilities within pharma organizations. Likely to initially have been placed in the hands of human resources and talent recruitment executives, LinkedIn is now a fully fledged communications vehicle. It goes without saying that a unified corporate message projected across all posted content is crucial. See also: Hints from the pros: Social media done well requires risk Takeda Pharmaceuticals is one organization undergoing such a transition. Caroline Onagan, associate director of Takeda's global talent acquisition operations, is the company's LinkedIn veteran, having clocked about six years of regular use for recruiting purposes. “For talent acquisition, we use LinkedIn end-to-end for all stages of the candidate lifecycle,” she explained. “We create awareness for those that don't know us, we engage those that do have familiarity and have shown some level of interest, and we convert the people we want to hire into employees.” A major part of this mission is branding the company as a career destination. “We regularly push out updates to show all of the different things that are happening at the company, whether it's news about acquisitions, information about some of the initiatives that we have or the partnerships we have,” she said. “But we also try to engage employees to push out what it means to work at the company. It's authentic content, and that's a big focus for us as we move forward on LinkedIn.” Onagan notes that she is also increasingly interviewing candidates via video and experimenting with reaching out to potential employees with video content about Takeda. If you're thinking this sounds more like corporate communications than recruitment, well, you'd be right. Onagan now has an entire communications team working with her. Leading the charge is Rob Scott, Takeda's digital transformation leader in global communications. Scott describes his position as a “uniting role” for social media and digital monitoring across the global organization. He focuses on four pillars: education, empowerment (governance/good guidance), growth and innovation. See also: Lack of expertise limits pharma's Facebook use Takeda's LinkedIn strategy has three foundations: consolidation, engagement and growth. “The first thing we're trying to do is understand how we can service the needs of a global organization in a local approach,” said Scott. “Next, it's about using the data from that global audience to understand how we can create content that the audience wants, so that we can educate them about our therapeutic areas and our business in a way that they want to hear. The third piece is about how we can grow LinkedIn into a key channel for us.” For Takeda, LinkedIn is very much a work in progress, particularly when it comes to melding together the corporate content and recruitment objectives. “We're taking a very data-led approach to building an understanding of the different ways that we can deliver content,” Scott continued, emphasizing the importance of social listening and monitoring. Regulatory considerations are obviously paramount with any pharma-generated social-media activity, and Scott leads a cross-function digital governance group with the objective of building “very, very simple guidelines, protocols and processes to ensure that we follow the regulatory requirements,” he said. Scott has plenty of advice for other companies looking to build out their LinkedIn activities. “The first golden rule is to engage a cross-functional group of people,” he said. “The second is to take a strong approach to understanding and listening to what your audience wants to hear and not necessarily just what you to say. The third is to test things and then learn from them.”
Via Plus91
« Internet est devenu la principale agora du débat public permettant aux gens de s’informer, d’échanger et donc de se forger une opinion. Donc, lorsqu’on aspire à restaurer ou à améliorer l’image d’une entité, l’enjeu est d’analyser sur Internet la manière dont se structurent les conversations. Pour restaurer l’image de la pharma, les thèmes du financement de la recherche, des études cliniques sur les médicaments ou encore de la structuration économique de la filière devront pouvoir être discutés publiquement », affirme Benoît Thieulin, La Netscouade.
Interview réalisée dans le cadre de la 27ème édition du Festival de la Communication Santé, novembre 2016.
En savoir plus, http://www.festivalcommunicationsante.fr/ et http://www.lanetscouade.com/
Via Festival Communication Santé
|

|
Scooped by
Rémy TESTON
April 22, 2017 4:14 AM
|
(AFP) - Le nouveau système d'auto-surveillance de la glycémie sans contact du laboratoire Abbott sera bientôt pris en charge à 100% par la Sécurité sociale en France, a annoncé vendredi la société dans un communiqué."Abbott vient d'obteni

|
Rescooped by
Rémy TESTON
from Social Media and Healthcare
April 11, 2017 2:31 AM
|
Pharma marketers continued to up their social media game in 2016, according to a new report, with many embracing disease awareness and charitable causes to drive engagement across social networks. Unmetric, a social media market intelligence company, analyzed the U.S. activity and consumer engagement of 15 big pharma brands in 2016, and it found that disease awareness was the most engaging topic across all social media and pharma companies. Specifically on Facebook, company news and milestones also captured consumer attention, while on Twitter and LinkedIn, charitable causes and health tips scored well with consumers. “When we looked at all the campaigns, content and hashtags that worked, the broad finding that came out was that all pharma companies saw the highest engagement when talking about the things that matter to their customers—most importantly the disease or condition they’re dealing with,” Lakshmanan Narayan, co-founder and CEO at Unmetric, said in an interview. “The maximum engagement came when they demonstrated empathy for what their customer and community is going through.” Using a weighted scoring system of likes, comments and shares to gauge engagement, Unmetric found some of the most engaging Facebook posts in 2016 were Eli Lilly's posts about its online program to save money on its insulin products; Novo Nordisk's information about the carbon footprint of diabetes products; Boehringer Ingelheim's efforts for Brain Awareness Week; and Bayer’s 360-degree view of its illuminated brand cross from its rooftop. On Twitter, Bayer’s announcement of its Monsanto acquisition intent scored high, while a Novo Nordisk post welcoming hundreds of new employees ranked high on LinkedIn. But while pharma has made social media strides, not every pharma brand has company accounts on all social media, according to Unmetric. All 15 brands it studied are on Twitter and 11 maintain corporate Facebook pages, but only three companies—Bayer, Boehringer Ingelheim and Eli Lilly—have Instagram accounts. Unmetric also tracks other metrics, such as number and types of posts, reach, impressions, and customer reply time on social media. When looking at hashtags, for instance, on Facebook, campaigns like Boehringer Ingelheim’s #HiddenHeartChallenge and Eli Lilly’s #EndALZ scored high, while #WeCanICan from Bristol-Myers Squibb and #CervicalHealthMonth from Merck also did well. “Three or four years ago, when we talked to a pharma company, they would be relatively reluctant to get onto social networks because of all the regulations,” Narayan said. “It’s interesting to see how far we’ve come since then with what brands now say and do on social networks, and in general, brands speaking for a cause is really what strikes a chord the most.” Source
Via Plus91

|
Rescooped by
Rémy TESTON
from Social Media and Healthcare
April 4, 2017 2:30 AM
|
Seventy percent of Internet use is now on mobile — and taking market share from other platforms. Adapted from a live presentation from Google NY’s Mobile Trend…
Via Plus91

|
Scooped by
Rémy TESTON
March 15, 2017 3:19 PM
|
Sanofi et Voluntis, la société française spécialisée dans les logiciels compagnons thérapeutiques, ont annoncé aujourd'hui un accord non exclusif visant à fournir des solutions numériques de titrage de l'insuline, avec une application de téléphonie mobile conçue pour contribuer à l'amélioration de la prise de décision et à l'autogestion du diabète de type 2 chez les personnes traitées avec de l'insuline basale.

|
Rescooped by
Rémy TESTON
from Pharma Hub
March 8, 2017 9:39 AM
|

|
Rescooped by
Rémy TESTON
from Digital Disruption in Pharma
March 4, 2017 3:45 AM
|
Working with UK firm Cambridge Cognition the company is piloting the app to monitor and assess cognitive function in patients with MDD, which affects around 350 million around the world. Nicole Mowad-Nassar, vice president of external partnerships at Takeda Pharmaceuticals USA, said: “This collaboration is part of our strategy to embrace new technology to better understand the patient experience and assist healthcare professionals in creating improved patient care pathways.” The small 30-patient trials of adults who have been prescribed an antidepressant will evaluate the app's feasibility and compliance, and aims to understand how a wearable's measures of mood and cognition compare to traditional neuropsychological testing and patient reported assessments.
Via Pharma Guy

|
Rescooped by
Rémy TESTON
from Social Media and Healthcare
February 16, 2017 1:12 AM
|
1. Medtronic (Corporate)
The medical device company, which develops insulin pumps for diabetes patients, has more than 16,000 followers. Wendy Blackburn, EVP of Intouch Solutions, said Instagram makes sense for diabetes marketing, since it has always been a disease category that is active on social media and tends to have a broad age range of patients.
2. Team Novo Nordisk (Corporate)
“Racing to inspire, educate and empower everyone affected by diabetes” is the tagline of Novo Nordisk's diabetes pro cycling team. This account has more than 73,000 followers and close to 2,000 posts.
3. #BreatheBoldly(Campaign)
In honor of the late Leonard Nimoy, who famously played Mr. Spock from “Star Trek,” Philips Healthcare and the COPD Foundation launched the #BreatheBoldly campaign to raise awareness of COPD in November. Celebrities such as actress Whoopi Goldberg, actor Vince Vaughn, and reality TV star Melissa Gorga posted videos on their Facebook and Instagram pages to show the challenges of suffering from the condition.
4. Flonase (Brand)
Facebook Health industry manager Danielle Salowski points to GlaxoSmithKline's over-the-counter allergy treatment Flonase as a good example of Instagram used well, showcasing vibrant seasonal imagery with related comments and information about how allergies affect people in the winter, spring, fall, and summer.
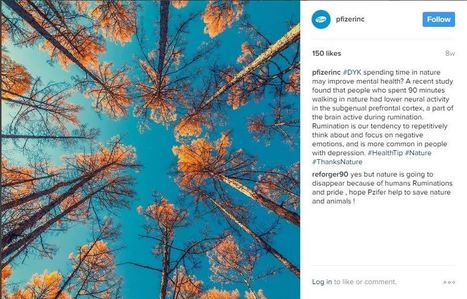
5. Pfizer (Corporate)
Blackburn doesn't recommend deleting comments, even when they are negative. She noted that Pfizer has a lot of trolls on its Instagram page, but doesn't delete them. “There will always be trolls and haters,” she said. “Deleting sends the wrong message because you're on there to engage to begin with.”
See also: How can drugmakers tell better stories? Try Instagram
6. Bayer4Animals (Corporate)
According to a study by BarkBox, dog owners in the U.S. on average post one photo or talk about their dog on social media six times a week. Bayer Animal Health targets pet owners and animal lovers with engaging animal photos and information about keeping them healthy.
7. Tylenol (Brand)
Like Flonase, Johnson & Johnson's Tylenol ties different moments throughout their year with content that tells a story through that lens, noted Salowski. “What I like about that is people know what to expect when they see content from Tylenol,” she said. “That's how they're connecting and engaging, and they just do a beautiful job with their photography and taking advantage of the mobile format.”
8. Emily Maynard for Diclegis (Influencer)
In June, The Bachelor and The Bachelorette's Emily Maynard Johnson made a post about her experience taking Duchesnay USA's morning sickness pill Diclegis — including safety information. It is the drugmakers second attempt at leveraging a celebrity spokesperson's Instagram handle — following a less successful example with Kim Kardashian West in July 2015 — to reach its target audience of expectant mothers.
9. Novartis (Corporate)
Novartis' corporate Instagram page comprises a mix of images and videos showing their corporate history and milestones, social and humanitarian efforts, and the drugmaker's attention to education and training.
10. Sanofi (Corporate)
There are drugmakers like Sanofi that have created an Instagram handle but not yet activated it with posts.
Via Plus91
The German life sciences company plans to bring its accelerator program for digital health startups to San Francisco. It's now available in Berlin, Moscow, and Shanghai among other places.
Via Lionel Reichardt / le Pharmageek

|
Scooped by
Rémy TESTON
February 11, 2017 3:04 AM
|
Pharmaceutical giant GlaxoSmithKline and MIT Connection Science have launched a new flu app called Flumoji to help users track symptoms & share that information with researchers working to improve disease surveillance.
Cases of the flu have increased nationally as the season begins to hit its usual peak. Tracking the activity of such an ubiqutious disease can help public health officials guide limited resources to the areas where they could get the most bang for their buck.
We’ve seen interesting uses of digital health for flu tracking in recent years, ranging from medical apps to guide flu treatment to the use of social media and internet searches to track activity. Flumoji is an Android app that includes educational material about the flu as well as symptom tracking features, which includes collection of data already being captured by the phone:
…the Flumoji app tracks a variety of real-time data from a user’s phone in order to detect fluctuations in a user’s activity levels, social levels, and general routine. These fluctuations are used to predict whether a user is experiencing a flu-like outbreak. Real-time data is only collected during the flu season.

|
Scooped by
Rémy TESTON
January 31, 2017 1:20 AM
|
According to Facebook Health industry manager Danielle Salowski, it's the combination of reach, scale, and engagement that make Facebook and Instagram useful tools for healthcare marketers. Facebook reaches 1.7 billion people around the world per month; on mobile, it reaches more than one billion people every day. Similarly, there are 500 million people using Instagram every month and 300 million each day. One in every five minutes on mobile is spent on either of the social media platforms, Salowski noted.“When you think about that in context, there's actually a Super Bowl happening every single day on mobile in the U.S.” It goes without saying that marketers continue to fall over themselves to affiliate themselves with the Facebook juggernaut. There are four million active advertisers on Facebook and 500,000 on Instagram, with 98 of the company's top 100 advertisers using both platforms. While Salowski declined to share specific details about the presence of pharma and healthcare marketers, she said that opportunities for them are numerous, given the six million health-related groups on Facebook that together accommodate 70 million users. See also: 8 ways for pharma to improve the way it uses Twitter “We're getting there with pharma brands getting more comfortable on the [Facebook] platform,” Salowski added. Established about a year ago, Facebook Health is the social-media giant's newest industry team, comprising a mix of experts from pharma, healthcare, and digital media. It is staffed by Facebook veterans across a range of markets, including New York, Washington D.C., and Menlo Park, California. For pharma marketers hoping to more effectively reach audiences on Facebook and Instagram, Salowski offers the following ten tips from the Facebook health team. 1. PARENTS AND BABY BOOMERS ARE ACTIVE ON FACEBOOK Parents spend 1.3 times more time on Facebook mobile than those who are not parents. Some drugmakers may think that Facebook is not relevant to their audience, but Salowski said that parents and users over 45 years old are actually quite active on the platform. According to the company's findings, parents spend 1.3 times more time on Facebook mobile than those who are not parents. In addition, Facebook found that 82% of its 45-plus audience said that modern technology allows them to connect with friends and family easily. “We see new moms using groups a ton. When you think about that life stage, there are obviously lots of questions you have. You want to create a community to get answers,” Salowski explained. “We also see a lot of groups for caregivers. They have a loved one who's suffering from a certain condition and the groups become a way for them to vent.” 2. FACEBOOK AND INSTAGRAM: ONE PORTAL, TWO PLATFORMS With a common advertising infrastructure, advertisers only need to create a single ad for Facebook and Instagram deployment. Photo credit: Franklin Heijnen/Creative Commons As part of its effort to be marketer-friendly, Facebook has a common advertising infrastructure across all of the company's products. This means that advertisers only need to create a single ad for Facebook and Instagram deployment. “All the technology, targeting, and measurements live in one portal, so it's really easy for advertisers to start using Facebook for mass global reach across variants and different products,” Salowski said. Of course, advertisers with more flexibility can choose to tailor their ads to provide custom content for each platform.“For pharma, sometimes it can be so hard to just get that one asset approved, they may want to use that across both platforms,” Salowski continued. “What we want the brands to do is experiment, because there's not a one-size-fits-all.” 3. FEED-BASED ADVERTISING WITHOUT INTERRUPTION Since both Facebook and Instagram are feed-based products, all advertising is integrated in the user's feed. There are no pop-ups or interstitials. “When you think back in the day, people used to have really personal relationships with their physician and pharmacist. We got a little away from that, but we believe that Facebook can help bring that back and help pharma reconnect with people one-on-one,” said Salowski. 4. PERSONAL TARGETING AT SCALE At a basic level, Facebook can target users by age, gender, and device, but it can target by specific interests and locations as well. “We built an interest graph where we can target people based on things they're interested in, people they follow, and pages they like,” explained Salowski. “It's not just mass marketing being bought on television. Our targeting can get to the root of more about your patient than just age and gender.” 5. START WITH A BRANDED PAGE
Building a branded page provides drugmakers with a vessel for their media content and allows them to leverage Facebook advertising's capabilities. At the same time, It can serve as a tool for drugmakers to drive business outcomes. “Our goal is to work as a partner with [pharma companies] every step of the way and get from asset creation to talking about content strategy and helping them navigate med/legal review,” said Salowski. She points to Allergan's Facebook page for immunosuppressive agent Retasis as an example of a branded page done well. 6. UNBRANDED PAGES CAN CONNECT AND ENGAGE AstraZeneca's Save Your Breath community for patients suffering from COPD has nearly 100,000 members. “We've seen some brands build unbranded communities off their Facebook pages that become safe places for people to come together and connect,” said Salowski. “It's more about the condition they're suffering from.It's never actually about the drug.” To that end, AstraZeneca's Save Your Breath community for patients suffering from COPD has nearly 100,000 members. 7. BE CREATIVE WITH SAFETY INFORMATION
Bayer was the first pharmaceutical company to test Facebook's scrolling ISI capability within the newsfeed. The drugmaker is using the ISI capability on an ad for its bluetooth-enabled auto-injector Betaconnect. The ad includes a click-to-call feature that directs users to Bayer's nurse call center. While not a new ad product, Facebook's scrolling ISI allows advertisers to layer text on existing Facebook ad units. Drugmakers can stitch together a static or video ad with a scrolling video of all the text in the ISI. 8. DRUGMAKERS CAN DISABLE COMMENTS ON FACEBOOK PAGES Drugmakers have the option to disable comments on Facebook pages to avoid adverse event reporting. It's a change the company made with pharma in mind, Salowski noted.“There are clear ways to contact the brand that we feature. But if the user has a question or wants to connect with the company, they can do that through the page.” 9. BE IMMERSIVE WITH VIDEO A video for GSK's meningitis campaign garnered more than 63,000 views and nearly 2,000 likes to date. More than 100 million hours of video are watched on Facebook every day, and pharma advertisers who have brought their videos onto the platform have seen good results, said Salowski. In September, GlaxoSmithKline ran a campaign on Facebook to raise awareness of meningitis. One of its videos for the campaign garnered more than 63,000 views and nearly 2,000 likes to date. Drugmakers such as Pfizer are also starting to experiment with Facebook's more immersive products such as Canvas, which combines text, links, images, and videos for a full-screen mobile advertising experience. “Canvas is a product where you can activate sight, sound, and motion to take over the entire screen,” said Facebook Health's Sachin Nanavati at Digital Pharma East in Philadelphia in October. “The average dwell time on Facebook Canvas ads is 31 seconds.” And there's also Facebook Carousel, which allows users to scroll across several images and integrate video. “It's a good opportunity to tell a story on mobile,” said Nanavati. "Canvas and Carousel ads are appealing because of the opportunity that additional screen space provides to display the appropriate balance of information related to our medicines and their ability to engage and inform consumers in an interactive way," said Julie Thaler, Pfizer's director of digital strategy and data innovation, in a statement. 10. FACEBOOK OFFERS CONTENT, WHILE INSTAGRAM OFFERS INSPIRATION There are two different purposes for each platform, said Salowski. Facebook is about content discovery, while Instagram is a platform for inspiration. “When I go on Instagram, I'm following fashion designers, beauty artists, and fitness stars – those are the people who inspire me in my life,” she explained. “You're going to Facebook to seek information. You might want news or you might want to see what your friends are up to. When you think about that from a brand perspective, brands can have it all on both, especially if you're demanding mobile first.”
Via Plus91, Lionel Reichardt / le Pharmageek

|
Rescooped by
Rémy TESTON
from Digital Disruption in Pharma
January 13, 2017 1:55 AM
|
Flumoji is your health wizard. Tell it how you feel and it will magically learn how to help protect you from Flu and other ailments.
This MIT study is designed to help increase awareness of the spread of flu and flu-like symptoms and educate you on how to reduce the risk of -- and help prevent -- flu infection. Your data along with other users of the app could potentially improve overall health outcomes in the general population.
Flumoji is being tested by MIT and GSK to see if it can speed up identification of flu outbreaks. “Real-time tracking of seasonal flu outbreaks is key,” says GSK on Facebook. “However, researchers have yet to find a tracking mechanism that’s fast and reliable enough to support testing of potential #flu treatments in clinical trials.”
Via Pharma Guy
|




 Your new post is loading...
Your new post is loading...