 Your new post is loading...

|
Scooped by
Juan Lama
June 24, 10:48 AM
|
RetroVirox has launched a Summer Promotion with a 30% discount for antiviral and neutralization services against 4 viruses, including influenza, dengue, human metapneumovirus (HMPV) and respiratory syncytial virus (RSV). Discount applies to services initiated between July 1 and August 31 2025
Contact us at info@retrovirox.com for inquiries and additional info

|
Scooped by
Juan Lama
September 30, 11:10 AM
|
With some analyses generating signs of efficacy, the biotech made the case that the data support further development of the direct-acting antiviral. A phase 2b trial of Enanta Pharmaceuticals’ respiratory syncytial virus (RSV) drug candidate has missed its primary endpoint. But, with other analyses generating signs of efficacy, the biotech made the case that the data support further development of the oral, direct-acting antiviral zelicapavir. Investigators randomized 186 patients to receive zelicapavir or placebo once daily for five days. Enanta later narrowed its efficacy population to include just the 175 patients who tested positive for RSV at a central laboratory. After the treatment period, the biotech tracked participants for a further 28 days to assess the effect of zelicapavir. The time to resolution of RSV lower respiratory tract disease (LRTD) symptoms was statistically no better on zelicapavir than on placebo, causing the trial to miss its primary endpoint. Enanta reported a 0.5-day improvement over placebo in time to the complete resolution of the four LRTD symptoms in the efficacy population. Other analyses linked zelicapavir to bigger improvements in time to symptom resolution. In the efficacy population, all 13 RSV symptoms cleared up 2.2 days faster in the treatment cohort. When looking at all 29 parameters in a patient-reported respiratory infection tool, Enanta found symptoms resolved 3.6 days faster on zelicapavir. The biotech also shared analyses of how zelicapavir compared to placebo in patients who had congestive heart failure, chronic obstructive pulmonary disease or who were aged 75 years or older. In total, 81% of participants met one of the criteria for inclusion in the subgroup analyses. Limiting assessments to those patients, Enanta linked zelicapavir to a three-day improvement in the time to resolution of LRTD symptoms. Time to resolution of the 13 RSV symptoms was 6.7 days shorter on the study drug in the subpopulation. The difference when looking at all 29 parameters was 7.2 days. Enanta shared data on a secondary endpoint that used another scale and on the rates of hospitalization—1.7% for zelicapavir versus 5% for placebo—to further support its argument that the results are positive. That conclusion informed comments by Scott Rottinghaus, M.D., chief medical officer of Enanta, about the next steps. “We believe the totality of these data provides strong rationale for further clinical advancement of zelicapavir,” Rottinghaus said in a statement. “Importantly, we identified multiple potential registrational endpoints for a phase 3 trial.” In a note to clients, Evercore analyst Jonathan Miller lauded the drug's “robust benefit” and stated that “no one in [the] RSV space has ever shown benefit like this with an antiviral before.” Shares in Enanta surged by about 40% to $11.03 by early afternoon on Monday from a closing price of $7.90 on Friday. Enanta's Press Release (Sept. 29, 2025): https://ir.enanta.com/news-releases/news-release-details/enanta-pharmaceuticals-reports-positive-topline-results-its

|
Scooped by
Juan Lama
September 26, 12:59 PM
|
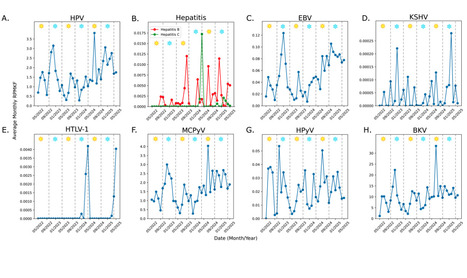
Background Oncogenic viruses cause high-risk cancers in humans and are responsible for nearly 20% of all cancer cases worldwide. Currently, very limited data exists in the realm of wastewater-based viral epidemiology (WBE) of cancer-causing viruses, with existing studies using targeted approaches (i.e PCR-based approaches) which lack scalability. Our study aims to carry out WBE with hybrid-capture probes to detect and track multiple oncogenic viruses simultaneously in wastewater across Texas, USA, overcoming the drawbacks associated with targeted approaches. Methods Here, we used a hybrid-capture approach to detect, filter and sequence oncogenic virus signals from wastewater samples collected over a duration of three years, from May 2022 to May 2025. Once viral reads were sequenced, we utilized established computational tools to characterize reads into their respective virus of origin. Next, viral abundances of each characterized oncogenic virus were tracked over time and read coverage across their genomes was measured using read mapping techniques. Findings We detected six known oncogenic viruses, along with three suspected oncogenic viruses across all sampling locations within Texas. Over three years, viral abundance gradually increased, with distinct peaks and dips over the summer and winter months. The prevalence of high-risk viruses such as HPV and EBV rose sharply, with increases in abundance observed post-2024. We also obtained nearly 100% genome coverage with viral reads captured using a hybrid-capture technique for almost all oncogenic viruses and their types. Interpretations Our study shows that a hybrid-capture method can efficiently overcome the challenges faced with using targeted approaches for WBE. Using this method, we get broader read coverage, coupled with concurrent and consistent real-time tracking dynamics of multiple oncogenic viruses. Our findings also emphasize the persistent circulation and rising prevalence of high-risk cancer-causing viruses, underscoring the need for sustained public health interventions to protect communities and assess viral prevalence in high-risk populations. Preprint in medRxiv (Sept. 18, 2025): https://doi.org/10.1101/2025.09.17.25335998

|
Scooped by
Juan Lama
September 24, 11:45 AM
|
A drug that provides near-perfect protection against H.I.V. with shots just twice a year will be made available at $40 per patient annually in low- and middle-income countries, offering new hope for ending the H.I.V. epidemic. Although current treatments can suppress H.I.V., there is no real cure, making affordable prevention crucial for turning back the virus. About 1.3 million people worldwide become infected with H.I.V. every year. The drug, lenacapavir, is given as two shots every six months. Through two deals announced on Wednesday by philanthropic organizations, the shots would cost the same as daily oral pills to prevent H.I.V., making lenacapavir a realistic choice in countries with constrained resources....

|
Scooped by
Juan Lama
September 19, 12:51 PM
|
Purpose Early detection of HPV-associated oropharyngeal cancer (HPV+OPSCC), the most common HPV cancer in the United States, could reduce disease-related morbidity and mortality, yet currently, there are no early detection tests. Circulating tumor HPV DNA (ctHPVDNA) is a sensitive and specific biomarker for HPV+OPSCC at diagnosis. It is unknown if ctHPVDNA is detectable prior to diagnosis, and thus it’s potential as an early detection test. Methods Plasma samples from the MassGeneralBrigham biobank collected 1.3-10.8 years prior to diagnosis from HPV+OPSCC patients (n = 28) and age- and sex-matched controls (n = 28) were blinded and run on a newly developed and validated multi-feature HPV whole genome sequencing liquid biopsy assay and a validated HPV antibody assay. Results ctHPVDNA results were positive in 22/28 pre-diagnostic samples from HPV+OPSCC cases (sensitivity 79%) with a maximum lead time of 7.8 years. ctHPVDNA results were negative in all controls (0/28 controls, 100% specificity). Diagnostic accuracy was highest within four years of cancer diagnosis and was higher than HPV Ab detection within the same time frame (p-value 0.004). Application of a machine learning model trained and tested on an independent cohort of 306 cases and controls increased the sensitivity of detection to 27/28 cases (overall sensitivity 96%) and the maximum lead time to 10.3 years. Conclusions Circulating tumor HPV DNA can be detected in the blood years prior to diagnosis with HPV+OPSCC, with high specificity, in a case-control cohort of 56 participants. ctHPVDNA detection alone, or in combination with previously identified serological biomarkers may be a feasible approach to early detection of HPV+OPSCC. Published Sept. (10, 2025): https://doi.org/10.1093/jnci/djaf249

|
Scooped by
Juan Lama
September 17, 12:07 PM
|
An immunocompromised man endured ongoing acute COVID-19 for more than 750 days. During this time, he experienced persistent respiratory symptoms and was hospitalized five times. In spite of its duration, the man's condition differs from long COVID as it wasn't a case of symptoms lingering once the virus had cleared out, but the viral phase of SARS-CoV-2 that continued for over two years. While this record may be easy to dismiss as something that occurs only to vulnerable people, persistent infections have implications for us all, US researchers warn in their new study. "Long-term infections allow the virus to explore ways to infect cells more efficiently, and [this study] adds to the evidence that more transmissible variants have emerged from such infections," Harvard University epidemiologist William Hanage told Sophia Abene at Contagion Live. "Effectively treating such cases is hence a priority for both the health of the individual and the community." Boston University bioinformatician Joseline Velasquez-Reyes and colleagues' genetic analysis of viral samples collected from the patient between March 2021 and July 2022 revealed what the virus was up to during its extended invasion. The virus's mutation rate within the patient ended up similar to that usually seen across a community. What's more, some of these mutations were awfully familiar. Spike mutations matched positions of those seen in the omicron variant of SARS-CoV-2, for example. Within just one person, the same types of mutations that led to the emergence of the faster multiplying omicron variant were on their way to being repeated. This backs the theory that omicron-like changes developed from selection pressures the virus experiences inside our bodies, the researchers explain. The patient, who has advanced HIV-1, believes they contracted SARS-CoV-2 in mid-May of 2020. During this time, he was not receiving antiretroviral therapy, nor able to access the necessary medical care despite suffering from respiratory symptoms, headaches, body aches, and weakness. The 41-year-old had an immune helper T-cell count of just 35 cells per microliter of blood, explaining how the virus managed to persist for so long. The healthy range is 500 to 1,500 cells per microliter. Luckily, in this case at least, the stubborn invader was not highly infectious. "The inferred absence of onward infections might indicate a loss of transmissibility during adaptation to a single host," Velasquez-Reyes and team suspect. Still, there's no guarantee other infections that establish long-term camps inside us will follow the same evolutionary path, which is why experts are wary and calling for continued close monitoring of COVID and adequate access to healthcare for everyone. "Clearing these infections should be a priority for health-care systems," the researchers conclude. To reduce the chances of problematic mutations, physicians and researchers urge communities to keep up vaccinations and continue masking in crowded, enclosed areas. Research published in TheLancet (Septmember 2025): https://doi.org/10.1016/j.lanmic.2025.101122

|
Scooped by
Juan Lama
September 15, 11:40 AM
|
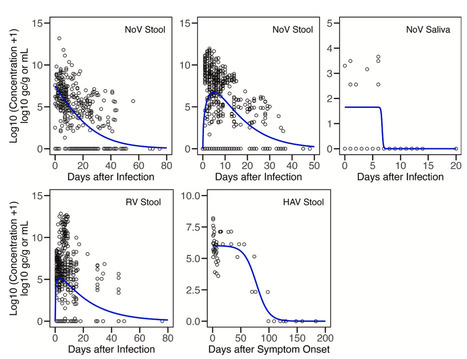
Background Wastewater-based epidemiology can inform the understanding of infectious disease occurrence in communities. Quantitative information on shedding of pathogen biomarkers in excretions that enter wastewater is needed to link measurements of pathogen biomarkers to rates of disease occurrence. Methods We compile, summarise, and compare data on shedding of human norovirus, rotavirus, hepatitis A virus, and adenovirus group F in stool, vomit, urine, saliva, mucus, and sputum using a systematic review and meta-analysis approach. Findings We provide summaries of measured concentrations of the viruses across excretions where data exist. We provide longitudinal shedding profiles in terms of concentrations and positivity rates. Duration of shedding and day of peak shedding are also provided. Interpretation There are limited data available for excretions other than stool, and limited data available for adenovirus group F. The aggregated data provided herein can serve as model inputs to translate wastewater enteric virus biomarker concentrations to disease occurrence rates. The study highlights data gaps and research needs. Published in eBiomedicine (August 5, 2025):

|
Scooped by
Juan Lama
September 11, 12:09 PM
|
A mix of three antibodies seems to protect mice against several strains of influenza and could one day be useful against seasonal flu or pandemics. A cocktail of antibodies could give us a new weapon to fight seasonal flu and new strains that cause pandemics. The mix protected mice from various strains of influenza, but hasn’t yet been tested in humans. Most flu treatments and vaccines rely on prompting the body to make proteins called neutralising antibodies. These bind to specific strains of a virus, preventing it from infecting cells. Such medical interventions can be very effective, but can take many months to develop and may lose effectiveness if the virus mutates. This is why flu vaccines are updated seasonally and why researchers are working on a universal vaccine that would protect against all flu strains or even against all viruses. Silke Paust at the Jackson Laboratory in Farmington, Connecticut, and her colleagues have a different approach. They are focusing on non-neutralising antibodies, another kind of protein produced by the immune system. Researchers have largely ignored these proteins for fighting infectious diseases because they don’t prevent infection. Instead, they empower the immune system to kill the virus responsible by tagging already infected lung cells. “We are making a therapy, not a vaccine. What we are trying to do is create a drug that you can give prophylactically or therapeutically after infection to prevent severe disease and death,” says Paust. Paust and her colleagues focused on antibodies that would target an influenza virus protein in a region called M2e, which is essential for the virus to replicate itself and is nearly unchanged in all flu strains. The researchers conducted a series of experiments in which they tested how well the antibodies worked individually or in combination in mice that were infected with a flu virus, and found that combining three antibodies gave the best results. They tested the cocktail in mice exposed to two strains of H1N1 influenza, including the one that caused the 2009 swine flu pandemic and gave rise to the currently circulating H1N1, and two avian influenza strains: H5N1, which is infecting wildlife around the world and some livestock in the US, and H7N9, which can be deadly to both humans and other animals. The researchers discovered that the cocktail reduced disease severity and the amount of virus in the lungs, and improved survival rates in both healthy and immunocompromised animals. For H7N9, for example, all mice survived when given the antibody cocktail in the first three days after infection, 70 per cent survived if treated on day four and 60 per cent if treated on day five. It is the first time we have seen such broad protection against flu in living animals, says Paust. The cocktail also worked when given before infection, so the drug could potentially be used in advance to prevent illness. Even after 24 days of treatment, there were no signs of the virus successfully mutating to resist it. “If the virus wants to mutate away from the therapy, it would have to evade all three antibodies because they don’t bind in exactly the same way,” says Paust. “As a proof of principle, this shows how a cocktail of antibodies might be utilised as a drug to treat people during a flu pandemic which could be used in parallel to vaccines,” says Daniel Davis at Imperial College London. “But this would need to be tested in humans before this can be considered a true medical advance.” The next step, says Paust, is to alter the antibodies that target M2e to make them look like human proteins, so the immune system doesn’t see them as invaders and attack them, which has been done before with many antibodies. If that works, safety and efficacy trials would follow. Paust envisions the cocktail being used as a stockpiled drug to fight seasonal flu outbreaks. “Ideally, this would be something to give to high-risk patients at the beginning of the season,” she says. “It would mean that they wouldn’t get very ill, essentially.” Research Published in Science Advances: https://www.science.org/doi/10.1126/sciadv.adx3505

|
Scooped by
Juan Lama
September 8, 11:34 AM
|
He was only 37 when he made a discovery that challenged the existing tenets of biology and led to an understanding of retroviruses and viruses, including H.I.V. David Baltimore, a biologist who in 1975 won a Nobel Prize for a startling discovery that seemed to rock the foundations of the fledgling field of molecular biology, died on Saturday at his home in Woods Hole, Mass. He was 87. The cause was complications of several cancers, his wife, Alice Huang, said. Dr. Baltimore was only 37 when he made his Nobel-winning discovery, upending what was called the central dogma, which stated that information in cells flowed in only one direction — from DNA to RNA to the synthesis of proteins. Dr. Baltimore showed that information can also flow in the reverse direction, from RNA to DNA. The key was finding a viral enzyme, called a transcriptase, that reversed the process. The discovery led to an understanding of retroviruses and viruses, including H.I.V., that use this enzyme. Today, gene therapies with disabled retroviruses are used to insert good genes into patients’ DNA to correct genetic diseases. Admired and envied, lionized and attacked, Dr. Baltimore spent most of his life in the scientific limelight, a towering figure of modern biology. He was president of two leading universities and an early proponent of AIDs research; he also fought what turned out to be trumped-up charges of fraud in a highly publicized decade-long case, beginning in the 1980s, involving accusations that a researcher in his lab had misreported data. In 1968, Dr. Baltimore joined the faculty of the Massachusetts Institute of Technology. Two years later, he began the work that would win him the Nobel Prize. It was a time when a group of Young Turks ruled the M.I.T. biology department. Dr. Baltimore was most definitely one of them, with a coterie of graduate student aspirants who hung onto his every word and vied to work in his lab. “Most of us young faculty at M.I.T. were thought of as arrogant,” his friend David Botstein, a former Princeton professor, said in an interview for this obituary. “David fit into that culture of competitive smartness. He was the smartest of all.” Dr. Baltimore first presented his data overthrowing the central dogma at an evening seminar in an M.I.T. classroom, inviting just faculty and friends. Dr. Botstein was there. “I remember it like it was yesterday,” Dr. Botstein said. “It was in room 16-310. He gave this talk and I remember walking out of it and saying to Maurie Fox” — another faculty member — “‘He is going to get the Nobel Prize for that.’” A few years later, it happened. Dr. Huang, an accomplished biologist who was working with Dr. Baltimore in his lab when he made the prizewinning discovery, was among the first to know. In 1975 she was at a conference in Copenhagen where George Klein, a scientist who was scheduled to give a talk, suddenly announced that he had been with a committee that decided on Nobel Prizes. In half an hour, Dr. Klein said, the committee would announce that Dr. Baltimore had won the Nobel Prize for Physiology or Medicine, along with two others: Howard Temin, who had independently made the same discovery, and Renato Dulbecco, for his work on tumor viruses. Dr. Huang “immediately got on the phone and called me,” Dr. Baltimore said in an interview for this obituary. He speculated that he was probably “the only person who ever was told he had won a Nobel Prize by his wife.” David Baltimore was born on March 7, 1938, in Manhattan to Richard and Gertrude (Lipschitz) Baltimore. His parents moved to Great Neck, N.Y., on Long Island, when he was in second grade so he and his younger brother, Robert, could attend better schools......

|
Scooped by
Juan Lama
September 5, 1:47 PM
|
4 September 2025 — The Democratic Republic of Congo (DRC) has declared its 16th outbreak of Ebola virus disease (EVD), confirmed in Kasai Province, government officials announced today. The index case is a 34-year-old pregnant woman admitted to hospital last month with symptoms including high fever and repeated vomiting. To date, 15 deaths have been reported, and 28 suspected cases identified across two health zones (Bulape and Mweka) in Kasai province. Four of the cases were among healthcare workers. Laboratory tests have confirmed the Zaire strain of Ebola. Investigations are ongoing to determine the source of exposure. Kasai province last reported an Ebola outbreak in 2008, while Equateur province experienced one in 2022. Africa CDC immediately engaged with the Ministry of Health and stands in close solidarity with the DRC. Following the declaration of the outbreak, Director General Dr Jean Kaseya travelled to the country and met with the Minister of Health to discuss outbreak management. Africa CDC has rapidly deployed experts to reinforce surveillance, contact tracing, data management, laboratory capacity, and infection prevention and control in the affected zones. “Africa CDC stands firmly with the people of the Democratic Republic of Congo. I have met with the Minister of Health to coordinate an urgent response, and we are taking strong measures to bring this outbreak under control — protecting communities and supporting the health workers on the frontlines,” said Dr Jean Kaseya, Africa CDC Director General. EVD is a zoonotic viral haemorrhagic fever affecting humans and non-human primates. The virus is transmitted from infected wild animals (such as fruit bats, porcupines, and primates) to humans. Human-to-human transmission occurs through direct contact with blood, bodily fluids or tissues of infected individuals, or contaminated surfaces and materials. Symptoms include fever, fatigue, muscle pain, headache, vomiting, diarrhoea, and unexplained bleeding or bruising. The disease has an average case fatality rate of about 50%. Africa CDC reaffirms its commitment to supporting the Democratic Republic of Congo in the fight against this outbreak. We will continue to strengthen and digitalize surveillance to ensure early detection, efficient tracing and effective case management. At the same time, we will protect healthcare workers on the frontlines through robust infection prevention and control measures, as well as vaccination, based on the country’s needs and in close coordination with partners. The Africa Centres for Disease Control and Prevention (Africa CDC) is a public health agency of the African Union. It is autonomous and supports member states in strengthening health systems. It also helps improve disease surveillance, emergency response, and disease control. Learn more at: http://www.africacdc.org and connect with us on LinkedIn, Twitter, Facebook and YouTube For more information and media inquiries Margaret Edwin | Director of Communication and Public Information | Africa CDC EdwinM@africacdc.org

|
Scooped by
Juan Lama
September 2, 12:25 PM
|
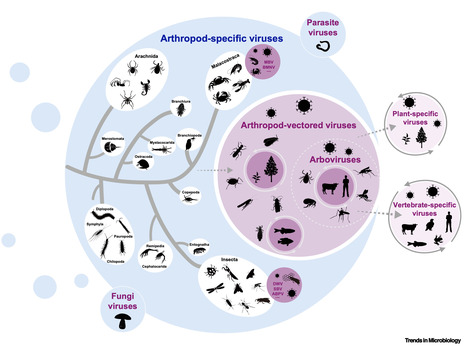
Highlights Our current understanding of arthropod virus diversity is biased towards viruses infecting humans or animals of economic and social importance. Arthropod viruses can cause diseases in arthropods, plants, vertebrates, and humans. Arthropod-specific viruses are likely ancestral to disease-causing arthropod-vectored viruses. Arthropod viruses are of ecological significance by directly exerting regulatory effects on arthropod populations and by indirectly regulating animal and plant populations. Abstract Research on arthropod viruses initially focused on those associated with diseases in vertebrates, particularly humans, as well as in plants of economic importance. However, the more recent deployment of metatranscriptomic sequencing of diverse arthropod species has facilitated the discovery of a multitude of novel arthropod viruses, in turn revealing that pathogenic viruses represent only a small component of the arthropod virome. In addition, arthropods may play a pivotal role in viral evolution and ecological dynamics, and have the potential to act as reservoirs for pathogens affecting vertebrates or plants. Due to active interactions between arthropod populations and diverse organisms – including fungi, plants, vertebrates, and even other arthropods in both aquatic and terrestrial ecosystems – there is an increased risk of the spillover of arthropod viruses to other organisms, including mammals. Herein, we review our current understanding of the diversity and ecology of arthropod viruses. We outline what is known about pathogenic arthropod viruses in diverse host types and emphasize the unique niche of arthropods as the source of emerging viral infectious diseases. Finally, we describe the evolutionary interactions between arthropod viruses and their hosts in ecosystems, at the same time highlighting their ecological significance with respect to regulating host populations. Published in Trends in Microbiology (August 2025)

|
Scooped by
Juan Lama
August 26, 12:32 PM
|
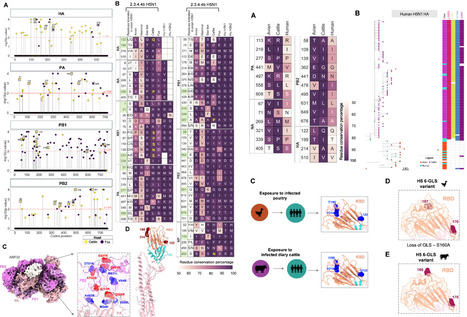
ABSTRACT The 2.3.4.4b clade highly pathogenic avian influenza H5N1 infected diverse non-human mammalian species, gained mammal-to-mammal transmission potential, and caused sporadic human infections. However, whether non-human mammals enable the human adaptation of 2.3.4.4b H5N1 to establish human infections is unclear. Gain-of-function research restrictions may hinder the assessment of 2.3.4.4b H5N1 human adaptations. Here, we tracked the evolution of 2.3.4.4b H5N1 that infected non-human mammals and evaluated their ability to gain human adaptations. The non-human mammal 2.3.4.4b H5N1 partly acquired classical human-adapting mutations, which are identical to the residues of H1N1pdm09 and seasonal human H3N2 viruses, while showing a few species-specific adaptations that might be potential barriers for successful human infections. The polymerase complex proteins, PA and PB2, acquired human adaptations in non-human mammals, with fox-infected viruses showing more positive selection in the polymerase complex. The human-adapting Q591K/R substitution in PB2 appeared only in the 2.3.4.4b clade but not in previously circulating H5N1 strains. Despite minimal changes in hemagglutinin (HA), A160T and T199I mutations near the receptor binding site of HA in dairy cattle viruses indicate the rapid HA glycan surface evolution affecting virus entry and immune evasion. The unbiased quantitative assessment of virus adaptations indicated that 2.3.4.4b H5N1 circulating in bears, cattle, dolphins, and foxes might show better human adaptive potential. Thus, 2.3.4.4b H5N1 appears to be acquiring human adaptations due to natural selection pressure in non-human mammals. Overall, our study delineates human adaptation and infection risk of specific non-human mammalian circulating 2.3.4.4b H5N1 strains. IMPORTANCE The 2.3.4.4b clade H5N1 virus emerged as a panzootic strain, leading to the unprecedented deaths of domestic and wild birds and diverse non-human mammalian species. Intriguingly, the 2.3.4.4b H5N1 transmitted to diverse mammalian species and gained mammal-to-mammal transmission, suggesting its pandemic potential. The H5N1 outbreaks in dairy cattle and sea lions are devastating, and they contributed to sporadic human infections. This indicates the ability of non-human mammal hosts, like dairy cattle, as potential sources for human transmission. However, the signatures of non-human mammal adaptations of 2.3.4.4b H5N1 and how these adaptations drive the human adaptive potential of 2.3.4.4b H5N1 are unclear. In this study, we show the specific molecular patterns of H5N1 proteins that determine species-specific adaptations in non-human mammals. We identified that 2.3.4.4b H5N1 circulating in non-human mammals is rapidly evolving with critical adaptations in PA, PB2, and HA and gaining human adaptive potential in specific non-human mammalian species. Published August 11, 2025:

|
Scooped by
Juan Lama
August 25, 12:31 PM
|
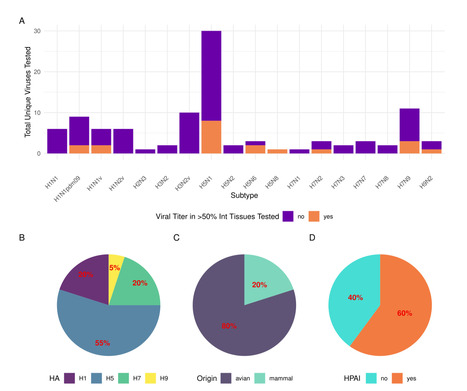
Influenza A virus (IAV) infection of the respiratory tract can cause both respiratory and non-respiratory symptoms. Gastrointestinal (GI) symptoms such as diarrhea, vomiting, and abdominal pain can occur in persons with seasonal influenza A or novel IAV infections, but the extent to which IAVs can infect and replicate in GI tissues is understudied. The ongoing outbreak of A(H5N1) IAV in US dairy cattle associated with sporadic human infections has highlighted the potential public health threat posed by the introduction of infectious virus into materials that may be consumed by humans, such as milk. Here, we review epidemiologic reports documenting the frequency of GI complications in humans infected with seasonal and novel IAVs and present laboratory studies supporting the capacity of IAV to replicate in mammalian GI tissues, with an emphasis on A(H5N1) viruses. Studies assessing the ability of IAV to cause mammalian infection following consumption of virus-containing material are also presented. Collectively, these studies suggest that gastric exposure represents a potential non-respiratory route for A(H5N1) IAVs in mammals that can lead to infection and support that IAV may be detected in mammalian intestinal tissues following multiple exposure routes. Published inmBio (August 12, 2025): https://doi.org/10.1128/mbio.01017-25
|

|
Scooped by
Juan Lama
September 30, 11:32 AM
|
Creating international viral biosafety guidelines are key to clearing up confusion, regaining trust and ensuring that essential research continues. In an opinion piece published in The New York Times in March, two leading virologists argued that experiments on a coronavirus found in bats and similar to those that cause Middle East respiratory syndrome (MERS) had been conducted without sufficient safety measures. The experiments involved infecting human cells with the live bat virus to see how the virus behaved. Regulatory authorities in many countries where research on potentially dangerous viruses is conducted, including the United States, the United Kingdom, France and Germany, would require such studies to be conducted in biosafety level (BSL) 3 or 4 facilities. (There are four biosafety levels, with BSL-4 being the most stringent.) But for these experiments — which were approved by the equivalent authorities in China — the 25 researchers across 7 institutes and universities in China (including the Guangzhou National Laboratory and the Wuhan Institute of Virology) used BSL-2 procedures. They also used a negative pressure ventilation system designed to prevent microorganisms from spreading outside the laboratory. In our view, this work and the discussion it has provoked highlights a broader and growing problem that the entire virology community needs to address. On the one hand, the threat posed by emerging infectious diseases is growing (see ‘A growing threat’), making investigations of potentially dangerous viruses more important. On the other hand, since the COVID-19 pandemic, trust in virology and science more broadly has declined and work on viruses has become more politicized. To improve trust in science — and to ensure that essential work on viruses can continue — international, standardized and transparent biosafety guidance is urgently needed. Here, we lay out how such guidance might be developed...

|
Scooped by
Juan Lama
September 26, 1:32 PM
|
In a groundbreaking advancement in virology, scientists have unveiled the elusive mechanism by which Epstein–Barr virus (EBV), a pervasive human herpesvirus, infects epithelial cells. This discovery centers on the identification of desmocollin 2 (DSC2) as the principal receptor facilitating EBV entry into epithelial cells—a critical insight that reshapes our understanding of EBV transmission and paves the way for novel therapeutic interventions. EBV, long recognized for infecting B lymphocytes and epithelial cells, has posed an enigma due to the inefficiency of cell-free infection in epithelial tissues despite their susceptibility in vivo. The research unravels this paradox, highlighting the importance of direct cell-to-cell contact and the pivotal role of DSC2 in enabling efficient viral spread. EBV is notorious for its dual tropism, infecting both B cells and the epithelial linings of the oropharynx. While infection of B cells has been extensively studied, epithelial infection mechanisms have remained less clear. Historically, EBV infection of epithelial cells using free viral particles was shown to be inefficient in laboratory settings, yet clinical manifestations suggest otherwise. Prior to this study, some candidates like EphA2 had been proposed as receptors facilitating epithelial infection, but their roles were inconsistent and failed to fully explain infection dynamics. The present study, utilizing an innovative genome-wide CRISPR-Cas9 screen, identified DSC2 as a receptor indispensable for the viral entry process in epithelial cells. The researchers conducted a comprehensive CRISPR screen aimed at pinpointing host factors that facilitate EBV entry into epithelial cells. This unbiased approach allowed them to systematically disable genes and observe the consequences on EBV infection efficiency. Through meticulous analysis, DSC2 emerged as a top candidate, with its knockout resulting in a significant decrease in viral infection rates. Intriguingly, desmocollin 3 (DSC3), a protein closely related to DSC2, was also implicated as a co-factor, not merely a redundant homolog. Together, DSC2 and DSC3 form a critical entry complex, necessary for both cell-free and — importantly — cell-to-cell contact infection modes. Building on these genetic insights, the team then employed loss- and gain-of-function experiments to validate DSC2’s role. Keratinocytes deficient in DSC2 and DSC3 showed a pronounced reduction in infection rates, both when exposed to cell-free viral particles and when co-cultured with EBV-infected B cells. Moreover, overexpressing DSC2 and DSC3 in receptor-negative cells significantly enhanced their susceptibility to infection, providing compelling evidence of their sufficiency and necessity. This dual requirement hints at a sophisticated viral entry mechanism optimized for the unique architecture of epithelial tissues, which are characterized by intricate cell-to-cell contacts. The therapeutic potential of targeting DSC2 was elegantly demonstrated by the application of monoclonal antibodies aimed at this protein. When epithelial cells were treated with antibodies directed at DSC2, EBV infection was markedly inhibited across a range of models, including normal oral keratinocytes, primary oral keratinocytes, and advanced head and neck epithelial organoids. The blockade effect became even more pronounced with a combination of antibodies against both DSC2 and DSC3, which efficiently suppressed the intimate cell-to-cell viral transfer that likely dominates natural infections. This points toward DSC2 as a highly promising target for preventative strategies, including vaccine development and antibody-based therapeutics. Mechanistically, DSC2’s interaction with the viral glycoprotein complex gH/gL was interrogated to unravel the intricacies of EBV fusion and entry. The study found that DSC2 directly binds to gH/gL, facilitating the membrane fusion process that enables viral capsid delivery into the host cytoplasm. This interaction is critical, as it orchestrates the structural rearrangements needed for EBV to breach the epithelial cell membrane. Interestingly, attempts to rescue infection in cells lacking DSC2 and DSC3 by overexpressing EphA2, a previously proposed EBV receptor, failed—highlighting a dependency hierarchy and confirming DSC2/3 as the dominant receptor complex for epithelial infection. The implication of these findings extends beyond basic virology, impacting our understanding of EBV-associated malignancies. EBV’s ability to exploit the DSC2 receptor complex for infection suggests that disruptions or variations in desmosomal components could influence susceptibility to infection and subsequent oncogenic transformation. Since EBV is causally linked to several epithelial malignancies, including nasopharyngeal carcinoma and certain head and neck cancers, targeting the DSC2 interaction axis holds potential not just for infection prophylaxis but also for interrupting oncogenic progression. Furthermore, the discovery refines the model of EBV pathogenesis within the oral cavity and oropharynx. The efficient transmission via direct B cell and epithelial cell contact underscores the significance of tissue architecture and cellular microenvironments in viral persistence and dissemination. EBV’s preference for this contact-mediated route rather than relying solely on cell-free virions explains longstanding clinical observations and reconciles previous inconsistencies in in vitro infection studies. This study thus bridges significant gaps in viral epidemiology and transmission dynamics. The application of advanced organoid culture systems in this research represents a leap forward in modeling EBV infection in near-physiological conditions. Head and neck epithelial organoids, which recapitulate the complex differentiation and stratification of epithelial tissues, allowed for more accurate assessment of viral entry and spread. The successful inhibition of infection in these organoids using DSC2-targeting antibodies strengthens the translational prospects of these findings, indicating that therapeutic strategies developed in vitro may be applicable in vivo. This study also raises fascinating questions about the broader role of desmosomal cadherins in viral infections. Desmocollins like DSC2 and DSC3 are key components of desmosomes, structures critical for cellular adhesion and tissue integrity. Viruses co-opting these proteins for entry point to a possible convergence of cell adhesion pathways and viral invasion mechanisms. This cross-talk may be exploited by other pathogens and represents a fertile ground for future research exploring host-microbe interactions at cellular junctions. Moreover, the elucidation of DSC2 as a primary receptor challenges prior paradigms that focused on other molecules such as integrins and Eph receptors. By highlighting a direct interaction with the viral glycoprotein complex, this study reorients therapeutic design toward desmosomal proteins, which may have been previously underappreciated. This shift in focus may inspire the generation of novel antiviral drugs that interfere specifically with the fusion process facilitated by DSC2-gH/gL binding. Given the ubiquitous prevalence of EBV and its association with a spectrum of diseases ranging from infectious mononucleosis to malignancies, the identification of DSC2 as the principal epithelial entry receptor offers a universal target. This could translate into the development of broadly applicable vaccines or monoclonal antibody therapies that prevent initial infection or limit viral spread, significantly impacting global health. In the future, clinical trials targeting DSC2 may redefine EBV management, shifting from symptomatic treatment to direct infection blockade. In summary, this pioneering work sheds light on the molecular underpinnings of EBV epithelial infection, introducing desmocollin 2 as the linchpin receptor that facilitates viral entry via direct cell-to-cell contact. The reliance on DSC2 and DSC3 for infection, the demonstrable blockade via antibodies, and the failure to rescue infection through alternative receptors compel a reevaluation of EBV biology. These discoveries have far-reaching implications for virology, oncology, and therapeutic development, marking a paradigm shift in the battle against this ubiquitous virus. The comprehensive investigation by Wang et al. not only clarifies the elusive mechanism of EBV epithelial infection but also inspires an array of future research directions. Understanding the structural basis of the DSC2-gH/gL interaction, exploring how desmosomal integrity influences EBV pathogenesis, and translating these findings into clinical applications are poised to transform the landscape of EBV prevention and treatment. This study epitomizes the power of integrative genomic screening and cellular modeling in unraveling complex viral-host interactions. As scientists continue to unravel the complexities of EBV’s interactions with its human host, the identification of desmocollin 2 as a principal entry receptor is a milestone achievement. It underscores the intricate interplay between viral evolution and host cell biology, revealing how viruses have adapted to exploit cellular machinery to ensure survival and propagation. This breakthrough serves as a blueprint for tackling other viral pathogens with similarly enigmatic infection mechanisms, showcasing how cutting-edge technologies can illuminate biological mysteries with profound clinical impact. Research published in Nat. Microbiology (2025): https://doi.org/10.1038/s41564-025-02126-0

|
Scooped by
Juan Lama
September 25, 11:44 AM
|
Huntington’s disease has been successfully treated for the first time using a gene therapy, which may be available in the US as soon as next year. An experimental gene therapy has become the first treatment to successfully slow the progression of Huntington’s disease. While the findings are still preliminary, the approach could be a major breakthrough and may even lead to new therapies for other neurodegenerative conditions, like Parkinson’s and Alzheimer’s. How does the therapy work? The treatment, called AMT-130, targets abnormal proteins in the brain that are responsible for the progression of Huntington’s disease. People with the condition have a genetic mutation that causes the normally-benign huntingtin protein to accumulate in toxic clumps inside brain cells, ultimately killing them. Over time, this leads to memory loss, difficulty walking, slurred speech and other symptoms. The experimental therapy, developed by the Dutch biotechnology company uniQure, stops the production of these mutant proteins. It does so by delivering genetic material to brain cells packaged inside a harmless virus. This material then directs cells to produce a small genetic molecule, called microRNA, which is designed to intercept and disable the instructions for producing the toxic protein. Think of it like a molecular stop signal. The shocking discovery that our gut microbiome drives ageing How and where is the treatment delivered? The treatment targets two brain regions first impacted by Huntington’s disease: the caudate nucleus and the putamen. Both are located deep inside the brain, so doctors use real-time brain scans to guide a thin catheter into them. The entire procedure takes 12 to 18 hours. One injection seems to be enough to permanently lower levels of mutant huntingtin in the brain...

|
Scooped by
Juan Lama
September 22, 11:45 AM
|
In a Q&A with STAT, Nobel winner Drew Weissman addressed concerns raised about Covid shots at a recent meeting of federal vaccine advisers. Drew Weissman Refutes RFK Jr. Adviser's Claims About Covid Shots. On Thursday and Friday, the Centers for Disease Control and Prevention held a meeting of its Advisory Committee on Immunization Practices, a key panel that gives the CDC’s director guidance about what vaccines to recommend to the public. The panel, in the end, postponed its most controversial vote and made recommendations that seemed aimed at fostering doubts about the Covid-19 vaccines while still keeping them widely available. But some presenters and panelists raised concerns that the mRNA Covid vaccines — the ones made by Moderna and Pfizer/BioNTech — may have unconfirmed safety issues. In particular, Retsef Levi, who chairs ACIP’s working group on Covid vaccines, raised concerns that mRNA, the lipid nanoparticles they are encapsulated in, and the spike protein the vaccines produce may all persist and be widely distributed in the body. He also said they may provoke an immune response that is not understood and could even change the way the body reads its own genetic material. Moderna and Pfizer said that the claims were refuted by well-done studies that met global regulatory standards. STAT posed questions about these claims to Drew Weissman, a professor at the University of Pennsylvania and co-recipient of the 2023 Nobel Prize in physiology or medicine for his discoveries that enabled the creation of mRNA-based Covid-19 vaccines. That conversation, edited for length and clarity, follows. At the ACIP meeting, there were a lot of discussions about reports of mRNA being widely distributed in the body or persisting. If you look in the literature you can find papers that say that the earth is flat, you can find papers that say DNA isn’t double stranded. You can find anything. The problem is there’s thousands, tens of thousands, hundreds of thousands, millions of other papers that refute that. What these people do, is that they search, they find one paper or two papers that make an outlandish claim based on bad data that hundreds or thousands or tens of thousands of other papers refute, they don’t mention everything that refutes it. They only mention, oh, look, I found a paper that says spike is around for nine months! It’s just not true. Many good studies have not seen that. The RNA is gone in days. It doesn’t go to the brain. It doesn’t go to the eyes. What those studies did is they put huge doses of RNA into a mouse and used very sensitive assays, and that’s where it went. It goes everywhere. If you put a vaccine equivalent dose, you see it in the muscle, you see it in the draining lymph node, and that’s about it. But how certain are we that mRNA is not continuing to produce spike protein? For instance, the preprint authored by Akiko Iwasaki, which includes well-respected Yale researchers. That’s a paper that found at least residual spike protein. The problem is they used a bad assay to measure spike. It’s an ultra, ultra-sensitive assay, but it makes a lot of errors, and you don’t believe anything unless it’s above a certain level as real. What they reported was in the unknown area where it could just be noise in the assay versus real spike. Many other assays using good experiments, using good assays, don’t find spike protein circulating.
How sure are you? How sure are you that there is no case of mRNA being distributed more widely in the body? I know it’s not distributed widely. I mean, we showed that back in 2017 at vaccine doses. We’ve looked at mice, we’ve looked at macaques, we’ve looked at rabbits, and we’ve done as much as we could on humans at a vaccine dose. You don’t see RNA circulating in the placenta, in the testes, in the heart, in the eye, in the brain, all the places that they list. We and many others have looked and we just don’t see it. And the mRNA could not be persistent? Could one dose of mRNA continue to make spike protein for months in a rare patient? It is absolutely impossible. MRNA is degraded incredibly rapidly. When you modify it, it’s a little slower. It’ll last 24 hours. It never, ever lasts six months. That’s just impossible. But I can make an assay that gives you any answer you want, and that’s what these people have done. I’d like to read specifically from the concerns about mRNA vaccines made by Dr. Retsef Levi, the chair of the working group for ACIP. We covered his concern about “wide biodistribution and prolonged persistence of spike, mRNA and nano-lipid particles.” He also wrote that there are prolonged immune responses that are not understood.
In people who are vaccinated, it was incredibly rare to see that. It was mostly immunized mice. You can always find people who react differently. You can always find people who don’t respond, for instance. Another concern is a “frame shift leading to production of unintended proteins and related immune response,” meaning the vaccine has changed the way that DNA is read to make proteins. That was one paper that claimed that uridine caused a frameshift mutation and induced immune responses. It was never seen in thousands and thousands of other studies. What about the issue of DNA contamination — that DNA impurities are present at too high a level and that the lipoprotein that allows the mRNA to get into cells could lead to them being taken up? Just about every vaccine has DNA contamination. If it is made from eggs, if it is made from living cells, if it is an inactivated virus, if it’s a live virus. They’re all grown in cells and cells have DNA. So when you purify the virus, you get very low levels of DNA contamination. We’ve never seen an adverse event. And these are minute amounts. For mRNA, the DNA strands are a couple of nucleotides long. We’ve never seen them integrate into the chromosomes or cause cancer, it just hasn’t been seen. People give a milligram dose of DNA plasmid as a vaccine, it’s in clinical trials right now. We don’t see any adverse events from that. Is there anything else you’d like to say? What concerned me more from these meetings is they voted against combined childhood vaccines. They voted against making Covid-19 vaccines available to larger populations. They’re going to likely vote against giving hepatitis B vaccines to infants. So if you look back at the data, 250 years ago, 40% of kids never made it to adulthood. Today, it’s 4%. The majority of that decrease is due to vaccines. Vaccines have saved more lives than any other type of medical intervention, including antibiotics, including everything. Vaccines have saved an enormous number of lives. No vaccine was tested more extensively than the RNA vaccines, and no vaccine was given to more people than the mRNA vaccines, and they were found to be incredibly safe, safer than any other vaccine platform and effective. They saved 20 million lives, and they stopped a pandemic that was shutting down the world.

|
Scooped by
Juan Lama
September 18, 2:09 PM
|
Viral infectious diseases have caused millions of deaths worldwide. Antiviral agents are critical for controlling these infections; however, an open-access database dedicated specifically to antiviral agents remains unavailable. Here, we present AntiviralDB (https://www.antiviraldb.com/), an expert-curated resource that compiles both approved and experimental antiviral agents with laboratory-confirmed in vitro activity against a broad spectrum of human viruses. These include the human immunodeficiency virus, coronaviruses, hepatitis viruses, influenza virus, respiratory syncytial virus, herpes simplex virus, varicella-zoster virus, human cytomegalovirus, human papillomavirus, dengue virus, Zika virus, Ebola virus, mpox virus, norovirus, chikungunya virus, and 16 other common or life-threatening pathogens. Each antiviral agent in the database is annotated with key information, including its molecular target, in vitro antiviral activity (IC50, EC50, and CC50 across specific viral strains and cell lines), mechanism of action, and relevant pharmacokinetic and pharmacodynamic parameters. AntiviralDB also provides clinical efficacy and safety data derived from randomized clinical trials. Unlike existing drug databases, AntiviralDB offers two distinctive features: (i) standardized laboratory protocols for antiviral drug screening under appropriate biosafety conditions and (ii) clinical guidelines for the therapeutic use of antiviral agents against viral infections. By serving as a comprehensive repository of antiviral agents and their clinical applications, AntiviralDB aims to advance antiviral drug discovery and support the effective clinical management of viral infectious diseases. IMPORTANCE Over the past decade, viral infectious diseases have caused significant morbidity and mortality worldwide, underscoring the urgent need for effective antiviral therapies. However, there is a lack of an open-access database that consolidates detailed information on antiviral agents, clinical guidelines, and laboratory protocols. To address this need, AntiviralDB was developed to integrate extensive data on antiviral agents—including clinical efficacy, safety profiles, pharmacokinetic parameters, and in vitro and in vivo activities—together with standardized experimental protocols and clinical treatment guidelines. This resource empowers researchers and clinicians to (i) identify promising antiviral candidates for controlling infectious diseases, (ii) accelerate the discovery and development of novel therapeutics, (iii) optimize the clinical use of existing antiviral drugs, and (iv) enhance a quick response to emerging viral outbreaks. Published in Mbio (Sept. 11, 2025):

|
Scooped by
Juan Lama
September 16, 11:52 AM
|
Influenza A viruses (IAVs) have historically posed significant public health threats, causing severe pandemics. Viral host specificity is typically constrained by host barriers, limiting the range of species that can be infected. However, these barriers are not absolute, and occasionally, cross-species transmission occurs, leading to human outbreaks. Early identification of changes in IAV host specificity is, therefore, critical. Despite advancements, identifying host susceptibility from genomic sequences during outbreaks remains challenging. Timely predictions are critical for effective real-time outbreak management and risk mitigation during the early stages of an epidemic. To address this, we proposed Flu-level Convolutional Neural Networks (Flu-CNN), a model designed to analyze genomic segments and identify IAV host specificity, with a particular focus on avian influenza viruses that could potentially infect humans. Extensive evaluations on large-scale genomic datasets containing 911,098 sequences show that Flu-CNN achieves an impressive 99% accuracy in determining host specificity from a single genomic segment, even for high-risk subtypes like H5N1, H7N9, and H9N2, which have a limited number of viral strains. Given its high level of accuracy, the model was applied to identify key mutations and assess the zoonotic potential of these strains. Furthermore, our study presents a pioneering approach for predicting IAV host specificity, offering novel insights into the evolutionary trajectory of these viruses. The model’s significance extends beyond evolutionary analysis, playing a pivotal role in outbreak surveillance and contributing to efforts aimed at preventing the viral spread on a global scale. Published (August 22, 2025) in Human Genomcs: https://doi.org/10.1186/s40246-025-00812-y

|
Scooped by
Juan Lama
September 13, 12:08 AM
|
A child in Los Angeles County has died from a rare but always fatal brain disorder that develops years after a measles infection. Experts underscore the need for vaccination to protect the most vulnerable. The first dose of the vaccine is typically not administered until one year of age. Experts say the death underscores the need for high levels of vaccination in a population to protect the most vulnerable against the disease, as well as from side effects that can occur long after the initial illness has passed. “This case is a painful reminder of how dangerous measles can be, especially for our most vulnerable community members,” said Los Angeles County Health Officer Muntu Davis in a recent statement. The child who died suffered from subacute sclerosing panencephalitis (SSPE), a progressive brain disorder that usually develops two to 10 years after a measles infection. The measles virus appears to mutate into a form that avoids detection by the immune system, allowing it to hide in the brain and eventually destroy neurons. “It’s just a virus that goes unchecked and destroys brain tissue, and we have no therapy for it,” said Walter Orenstein, an epidemiologist and professor emeritus at Emory University, to Scientific American earlier this year. People with SSPE experience a gradual, worsening loss of neurological function and usually die within one to three years after diagnosis, according to the Los Angeles County Health Department. The disorder affects only about one in every 10,000 people who contract measles. But the risk may be as high as about one in 600 for those who are infected as infants. “There is no treatment for this. Children who suffer from this will always die,” said Paul Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia, in a previous interview with Scientific American. Offit, who had measles himself in the 1950s, has seen five or 10 cases of SSPE in his career. SSPE is one of several side effects of measles that go beyond the coughing, runny nose and characteristic rash of the original infection. Measles can also cause encephalitis, a faster-occurring brain inflammation, in one in every 1,000 people who are infected because the virus causes the immune system to attack a protein produced by certain brain cells. This inflammation kills about one in five people who develop it. Measles also causes “immune amnesia”: the virus seems to attack the immune system’s B cells, which remember previous pathogens the body has been exposed to, resulting in reduced immunity. There is some evidence this effect can last for a couple of years, making those who get measles more susceptible to other infectious diseases. These side effects are of particular concern because the measles virus is highly contagious—an order of magnitude more than seasonal influenza. With measles, viral particles emitted by coughing or sneezing can linger in a room for hours after the infected person has left. One infected person infects 15 more people on average. This year the U.S. saw its largest single measles outbreak since the disease was declared eliminated in 2000;the recent outbreak occurred mainly in Texas, New Mexico, Kansas and Oklahoma. Most of those infected were unvaccinated or had an unknown vaccination status. Of those infected, 12 percent were hospitalized, and three died of complications from the infection. The fatal cases included the first death of a child from measles in the U.S. in 22 years. Measles used to infect three million to four million people in the nation every year until vaccines became available in 1963. The measles vaccine is administered in two doses: typically, the first is given between 12 and 15 months of age and the second is given at four to six years. One dose is 93 percent effective at protection against infection, and two doses are 97 percent effective. Contrary to claims by Secretary of Health and Human Services Robert F. Kennedy, Jr., measles cannot be treated by vitamin A or cod liver oil. There is no cure or treatment for the disease beyond treatment of symptoms. The only effective means of combatting measles is widespread vaccination. At least 95 percent of a population must be vaccinated to prevent the spread of the disease and to protect either those who are too young to receive it or those who cannot be vaccinated because of other health conditions. “Infants too young to be vaccinated rely on all of us to help protect them through community immunity,” Davis said in his recent statement. “Vaccination is not just about protecting yourself—it's about protecting your family, your neighbors, and especially children who are too young to be vaccinated.”

|
Scooped by
Juan Lama
September 8, 12:19 PM
|
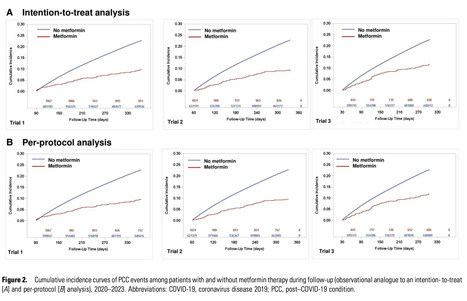
Background A subgroup analysis of the COVID-OUT trial's long-term outcome found that starting metformin within 3 days of coronavirus disease 2019 (COVID-19) diagnosis reduced post–COVID-19 condition (PCC) incidence by 63% in overweight or obese individuals. However, its generalizability remains uncertain. Objectives To evaluate the effectiveness of metformin in preventing PCC in adults with overweight or obesity who had a recent COVID-19 infection. Design A retrospective cohort study using a sequential target trial emulation framework. Data Sources The United Kingdom primary care data from the Clinical Practice Research Datalink Aurum database from March 2020 to July 2023. Participants Adults with overweight or obesity (body mass index ≥ 25 kg/m²) and a record of severe acute respiratory syndrome coronavirus 2 infection were included. Exclusions included metformin use in the prior year or metformin contraindications. Measurements The outcome was PCC, defined by a PCC diagnostic code or at least 1 World Health Organization–listed symptoms between 90 and 365 days after diagnosis, with no prior history of the symptom within 180 days before infection. The pooled hazard ratio and risk difference for the incidence of PCC were adjust for baseline characteristics. Results Among 624 308 patients, 2976 initiated metformin within 90 days of COVID-19 diagnosis. The 1-year risk difference for PCC in the intention-to-treat analysis was −12.58% (hazard ratio 0.36; 95% CI, 0.32–0.41), with consistent results in subgroup analyses. Limitations Findings may not apply to individuals with a normal body mass index. Conclusions Early metformin treatment in overweight or obese individuals may reduce PCC risk. Further research is needed to confirm causality and clarify metformin's role in PCC management. Published in Clinical Infect. Diseaseses (Sept.1): https://doi.org/10.1093/cid/ciaf429

|
Scooped by
Juan Lama
September 5, 2:13 PM
|
A pet cat in San Francisco had to be euthanized after contracting the bird flu virus after eating raw food, according to health officials. The U.S. Food and Drug Administration issued a warning on Wednesday after the San Francisco Department of Public Health reported a cat became sick with H5N1 bird flu after eating RAWR Raw Cat Food Chicken Eats brand raw cat food. The pet cat was euthanized after becoming ill. FDA officials then tested the cat’s food and found it was contaminated with H5N1 bird flu virus. Two other retail samples of the food were tested, and one of these samples tested positive for bird flu. Despite its name, bird flu can affect humans and other animals, including cats and dogs. Since 2024, when H5N1 started to be detected in dairy cows in the U.S., at least 70 people in the U.S. have developed the illness and one person has died, according to the U.S. Centers for Disease Control and Prevention. The American Veterinary Medical Association reports that “dozens of cats” (both domestic and wild) have developed H5N1 since 2024. Currently, it’s thought that the risk of cat-to-human transmission of bird flu is low, although people can develop the disease when handling raw food containing the virus. Signs of bird flu in a cat include “loss of appetite, lethargy, and fever” but can quickly progress to neurological symptoms like tremors, and discharge from the nose and eyes, severe depression, and respiratory symptoms like coughing. RAWR founder Sabrina Simmons posted a lengthy response to the FDA notification on the Like a Lion website, writing that the agency had not been forthcoming with information about the investigation. Simmons said there was not enough evidence the cat had died from H5N1. She also said the company removed the lots mentioned by the FDA weeks ago before the FDA posted the warning and that it was in full compliance with the FDA requirements for minimizing H5N1 risk. “Redirecting blame onto a single small manufacturer does not bring us closer to understanding avian flu, nor does it help prevent future issues in the food supply chain. This is an ongoing battle that raw food companies face,” Simmons wrote. FDA Warning (Sept. 3, 2025): https://www.fda.gov/animal-veterinary/cvm-updates/fda-notifies-pet-owners-tests-show-h5n1-contamination-certain-lots-rawr-raw-cat-food-chicken-eats

|
Scooped by
Juan Lama
September 5, 1:30 PM
|
Raising millions upon millions of disease-fighting mosquitoes per week is no easy task, Nature learnt during its visit to the facility. Curitiba, Brazil When biologist Antonio Brandão tells people that he works at a mosquito factory, they are often baffled. “Why would you make more mosquitoes?”, he recalls people asking. “We have enough of them.” But once he explains that the laboratory-raised insects can help to stop the spread of dengue — which strikes hundreds of thousands in Brazil each year and causes fever, headache and bone pain — they come around. Brandão is the production manager at not just any mosquito factory, but the world’s largest, located in the southern Brazilian city of Curitiba. Launched in July, the facility is expected to produce 100 million eggs a week from the mosquito Aedes aegypti. However, unlike wild A. aegypti, the main transmitter of dengue virus, those churned out by the factory carry a harmless Wolbachia bacterium that curbs the insects’ ability to spread viruses including dengue and Zika. The idea is to release the modified mosquitoes, which researchers call wolbitos, into cities in Brazil, where they will mate with their wild counterparts and the females will pass the bacterium on to their offspring, gradually converting the local population. The wolbito strategy, which is being spearheaded by the non-profit World Mosquito Program (WMP), has already shown success in Colombia, Indonesia and at home: in the Brazilian city of Niterói in the southeast, dengue cases dropped by 69% in areas where Wolbachia-carrying mosquitoes were released, compared with areas where they weren’t1. Brazil’s federal government has adopted the approach to fight dengue infections — which surged to a record 6.5 million confirmed cases in the country last year — alongside other preventive measures such as vaccines. However, researchers at the factory have discovered that raising millions of the mosquitoes is surprisingly tricky, especially because the insects are fussy about temperature and other factors. Nature visited the Wolbito do Brasil facility — run by a firm formed by the WMP, the Molecular Biology Institute of Paraná and Fiocruz, a research institute affiliated with Brazil’s health ministry — to hear first-hand about the lessons learnt by staff members before they released the factory’s first mosquitoes in late August. Their hard-won knowledge will inform other efforts to rein in mosquito-borne illnesses around the world, says Gabriela Paz-Bailey, an epidemiologist at the US Centers for Disease Control and Prevention who is based in San Juan, Puerto Rico. “There will be plenty of lessons learned from the Brazilian government involvement in this strategy.” A factory abuzz with activity Female A. aegypti mosquitoes need only a small amount of water — a puddle inside an abandoned tyre or bottle cap, for instance — to lay their eggs. This makes it difficult to prevent mosquitoes from breeding in urban areas, but it can also be used to the factory’s advantage. The facility has engineered small, dissolvable capsules, similar to those used for holding medicines, to store around 500 mosquito eggs each. These can be shipped to locations where they are needed, then easily dissolved in small amounts of water for hatching. Fish food is included in the capsules to nourish larvae. To produce such a large number of eggs in the first place, the factory relies on millions of adult mosquitoes mating and laying eggs around the clock. Researchers ensure that the insects carry a strain of Wolbachia, which can be passed from female mosquitoes to their offspring. Step inside Wolbito do Brasil with reporter Mariana Lenharo as she learns how the factory breeds and cares for its modified mosquitoes In one of the most impressive rooms at the facility, 66 mesh cages big enough for a human to stand inside hold roughly 10 million mosquitoes in total. Females lay their eggs on strips of paper placed at the bottom of each cage. The tiny black eggs, no bigger than a grain of sand, are collected, and either put into capsules or held for hatching at the facility, to replenish the insects in the cages. Getting mosquitoes hatched from the eggs to develop inside the controlled facility has been a challenge, however. At each stage of their life cycle, A. aegyptihave specific temperature and humidity requirements. Researchers store eggs in a cool but humid room to prevent them from drying out. Larvae, however, require warmer conditions, meaning that the facility needs to shuffle insects to other climate-controlled areas as they develop. “They are very delicate. If you vary some parameters of humidity and temperature, this affects them and impacts their productivity,” says Marlene Salazar, a biologist at the facility....

|
Scooped by
Juan Lama
August 27, 11:39 AM
|
The mammary gland is an essential organ for milk production, providing essential immune and nutritional support to offspring and supplying dairy products for human consumption. In both humans and animals, the lactating mammary gland is susceptible to bacterial and viral infections, which can lead to mastitis and, in some cases, vertical transmission to offspring, with potential adverse effects on infant health. However, until recently, the role of respiratory viruses in mammary gland infection has been relatively understudied, particularly their ability to infect mammary epithelial cells and transmit through lactation. The recent emergence of highly pathogenic avian influenza H5N1 clade 2.3.4.4b in dairy cattle has demonstrated the virus’s capacity to replicate in the mammary gland, cause mastitis, and produce high viral loads in milk. This raises significant concerns about the potential for zoonotic transmission to humans and other animals in contact with infected dairy cows and unpasteurized milk. In this mini-review, we highlight key studies that demonstrate the replication of influenza and other viruses in the mammary gland, summarize recent findings from experimental and natural H5N1 clade 2.3.4.4b infections in dairy cows and small animal models, and discuss the broader One Health implications of the current H5N1 outbreak. We emphasize the urgent need for an interdisciplinary collaboration across sectors to mitigate the risks posed by influenza viruses with pandemic potential. Published in J. Virology (August 12, 2025): https://doi.org/10.1128/jvi.01940-24

|
Scooped by
Juan Lama
August 26, 12:12 PM
|
The White House has denied reports that the government could soon ban COVID-19 vaccines, noting that in the absence of an official announcement, “any discussion about HHS policy should be dismissed as baseless speculation. The Daily Beast spoke to Aseem Malhotra, a British cardiologist and top adviser to the Make America Healthy Again Action, who said that many of the people closest to Kennedy have told the Secretary that they “cannot understand” why the U.S. continues to administer the vaccine. Several members of Trump’s family share this skepticism of the COVID-19 vaccine, according to Malhotra. The withdrawal of the coronavirus shots from the U.S. market could happen “in a number of stages,” according to Malhotra, which could include looking into vaccine safety data. “But given the increased talk of vaccine injuries in the past few weeks among the administration, it could also come with one clean decision.” In a statement to the Daily Beast, a spokesperson from the White House said the Trump administration continues to abide by “Gold Standard Science” and is “committed to radical transparency” regarding decisions affecting all Americans. “Unless announced by the Administration, however, any discussion about HHS policy should be dismissed as baseless speculation.” Since assuming his post in February, Kennedy has changed HHS policy to fit his decades-long history of vaccine opposition. In May, the Secretary pushed to have routine COVID-19 vaccination for healthy children and healthy pregnant women removed from CDC guidelines. A week earlier, FDA Commissioner Marty Makary, alongside Center for Biologics Evaluation and Research director Vinay Prasad, unwrapped a new risk-based framework for approving COVID-19 vaccines, focusing only on recipients who are at risk of the disease rather than attaining widespread vaccination. In June, Kennedy emptied the CDC’s Advisory Committee on Immunization Practices before reforming the committee days later with members of his choosing, some with anti-vaccine views that align with his own. Earlier this month, Kennedy scrapped more than 20 contracts under the Biomedical Advanced Research and Development Authority, which had been awarded to companies and organizations advancing mRNA vaccine research. “The data show these vaccines fail to protect effectively against upper respiratory infections like COVID and flu,” he said at the time without offering evidence to support that statement. Kennedy’s actions are drawing criticism and resistance from many quarters. Just last week, for example, the American Academy of Pediatrics continued to recommend COVID-19 vaccines for all children, regardless of risk profile, aged 6 to 23 months—a direct contradiction to the CDC’s guidelines. Similarly, hundreds of current and former HHS employees published an open letter last week, arguing that Kennedy is “complicit” in the erosion of the public’s trust in health authorities. This growing mistrust, according to the letter, culminated in the shooting incident earlier this month at the CDC headquarters in which one police officer was killed.

|
Scooped by
Juan Lama
August 25, 12:16 PM
|
Earlier this month, the FDA backed off on a pause in shipments of the chikungunya vaccine Ixchiq to older adults. Now, the regulator has reversed course. The FDA has suspended the approval of Valneva’s chikungunya vaccine Ixchiq due to “serious safety concerns” including at least one death that was “directly attributable to the vaccine.” The FDA noted in an announcement Friday that Ixchiq “appears to be causing chikungunya-like illness” in patients who had been inoculated. To date, the regulator has documented more than 20 reports of serious side effects consistent with chikungunya-like illness in recipients of the vaccine. Of these, 21 resulted in hospitalization and three led to death, including one from encephalitis that has been linked to the shot, per the announcement. According to a company release on Friday, the suspension is “effective immediately and requires Valneva to stop shipping and selling” Ixchiq in the U.S. Valneva’s shares declined 21% to $9.10 in pre-market trading Monday, compared to $11.64 at close on Friday. The FDA action caps a months-long rough patch for Valneva and Ixchiq. In May, the FDA and CDC recommended a temporarily halt on the use of the vaccine in people 60 years old and above amid “reports of serious adverse events, including neurologic and cardiac events.” At the time, the agencies had already flagged 17 serious complications in this age group, including two that have “resulted in death.” Then, earlier this month, the FDA lifted the pause, but recommended stricter labelling for the vaccine in this older patient subgroup. The FDA required Valneva to change Ixchiq’s prescribing information to say that the vaccine is only indicated for those who are “at high risk” of being exposed to the chikungunya virus. The label’s original language noted that Ixchiq was approved for those at “increased risk” of infection. Additionally, the FDA asked Valneva to include warnings that reflect these post-marketing findings for Ixchiq, including “adverse reactions that are consistent with severe complications of chikungunya, resulting in hospitalization, including encephalitis in a person who died.” Now, the shot has been pulled from the market. The agency’s “benefit-risk analysis broadly shows the vaccine does not have benefits outweighing risks, under most plausible scenarios,” the FDA said in its Friday update. Continued inoculation of patients “would pose a danger to health.” Ixchiq is a live attenuated virus shot that in November 2023 became the first FDA-approved chikungunya vaccine. The nod came via the regulator’s accelerated pathway, which requires Valneva to validate the shot’s clinical benefit in a confirmatory trial. In its announcement on Friday, the FDA alluded to the accelerated nature of Ixchiq’s approval, noting that “the clinical benefit of the vaccine has not yet been verified in confirmatory clinical studies.” Despite having the product pulled, Valneva will for now maintain its full-year 2024 guidance, as per its Friday release. In its second-quarter report earlier this month, the company said it expects product sales to hit €170 million to €180 million, or around $199 million to $210 million. In the first half of the year, Ixchiq brought in €7.5 million, or nearly $8.8 million. FDA Press Release (Updated August 25, 2025): https://www.fda.gov/safety/medical-product-safety-information/fda-update-safety-ixchiq-chikungunya-vaccine-live-fda-suspends-biologics-license-fda-safety Valneva's Press Release (August 25, 2025): https://valneva.com/wp-content/uploads/2025/08/2025_08_25_FDA_IXCHIQ_Update_PR_EN_Final2.pdf
|





 Your new post is loading...
Your new post is loading...




















https://recherchechimique.com/
https://recherchechimique.com/produit/acheter-victoza-en-ligne/
https://recherchechimique.com/produit/acheter-wegovy-en-ligne/
https://recherchechimique.com/produit/acheter-saxenda-en-ligne/
https://recherchechimique.com/produit/acheter-ozempic-en-ligne/
https://recherchechimique.com/produit/acheter-mysimba-en-ligne/
https://recherchechimique.com/produit/extase-molly/
https://recherchechimique.com/produit/bleu-et-jaune-ikea-mdma-220mg/
https://recherchechimique.com/produit/acheter-vyvanse-en-ligne/
https://recherchechimique.com/produit/brun-donkey-kong-mdma-260mg/
https://recherchechimique.com/produit/acheter-adderall-xr-en-ligne/
https://recherchechimique.com/produit/acheter-du-cristal-de-mdma-en-ligne/
https://recherchechimique.com/produit/acheter-du-marbre-hash-en-ligne/
https://recherchechimique.com/produit/acheter-3-meo-pcp-en-ligne/
https://recherchechimique.com/produit/acheter-acquista-xanax-2mg-en-ligne/
https://recherchechimique.com/produit/acheter-de-lheroine-en-ligne/
https://recherchechimique.com/produit/acheter-de-la-codeine-en-ligne/
https://recherchechimique.com/produit/acheter-de-la-methadone-en-ligne/
https://recherchechimique.com/produit/acheter-de-la-morphine-en-ligne/
https://recherchechimique.com/produit/acheter-hydrocodone-en-ligne/
https://recherchechimique.com/produit/acheter-oxycontin-en-ligne/
https://recherchechimique.com/produit/acheter-percocet-en-ligne/
https://recherchechimique.com/produit/ayahuasca-dmt/
https://recherchechimique.com/produit/bonbons-au-lsd/
https://recherchechimique.com/produit/buvards-lsd/
https://recherchechimique.com/produit/comprimes-de-gel-de-lsd/
https://recherchechimique.com/produit/cristaux-de-ketamine/
https://recherchechimique.com/produit/deadhead-chimiste-dmt/
https://recherchechimique.com/produit/glace-methamphetamine/
https://recherchechimique.com/produit/ketamine-hcl/
https://profarmaceutico.com/
https://profarmaceutico.com/Prodotto/acquista-mounjaro-online/
https://profarmaceutico.com/Prodotto/acquista-victoza-online/
https://profarmaceutico.com/Prodotto/acquistare-saxenda-6mg-ml-online/
https://profarmaceutico.com/Prodotto/acquista-ozempic-online/
https://profarmaceutico.com/Prodotto/acquista-wegovy-online/
https://profarmaceutico.com/Prodotto/acquista-mysimba-online/
https://profarmaceutico.com/Prodotto/acquista-targin-40-mg-online/
https://profarmaceutico.com/Prodotto/acquistare-targin-20-mg-10-mg/
https://profarmaceutico.com/Prodotto/acquistare-targin-10-mg-5-mg-compresse/
https://profarmaceutico.com/Prodotto/acquista-dysport-online/
https://profarmaceutico.com/Prodotto/acquista-neuronox-online/
https://profarmaceutico.com/Prodotto/acquista-bocouture-online/
https://profarmaceutico.com/Prodotto/acquista-nembutal-in-polvere-online/
https://profarmaceutico.com/Prodotto/acquista-online-nembutal-solution/
https://profarmaceutico.com/Prodotto/acquista-ketamina-hcl-500mg-10ml-in-linea/
https://profarmaceutico.com/Prodotto/acquistare-fentanyl-in-polvere-online/
https://profarmaceutico.com/Prodotto/acquistare-fentanyl-online/
https://profarmaceutico.com/Prodotto/acquista-cristallo-mdma-online/
https://profarmaceutico.com/Prodotto/a-215-ossicodone-actavis/
https://profarmaceutico.com/Prodotto/acquista-adderall-30mg/
https://profarmaceutico.com/Prodotto/acquista-adipex-online/
https://profarmaceutico.com/Prodotto/acquista-ossicodone-online/
https://profarmaceutico.com/Prodotto/acquista-oxycontin-online/
https://profarmaceutico.com/Prodotto/acquista-codeina-online/
https://profarmaceutico.com/Prodotto/acquista-adma-online/
https://profarmaceutico.com/Prodotto/acquista-ambien/
https://profarmaceutico.com/Prodotto/acquista-ativan-online/
https://profarmaceutico.com/Prodotto/acquista-botox-online/
https://profarmaceutico.com/Prodotto/acquista-cerotti-al-fentanil/
https://profarmaceutico.com/Prodotto/acquista-codeina-linctus-online/
https://profarmaceutico.com/Prodotto/acquista-demerol-online/
https://profarmaceutico.com/Prodotto/acquista-depalgo-online/
https://profarmaceutico.com/Prodotto/acquista-diazepam-online/
https://profarmaceutico.com/Prodotto/acquistare-idromorfone-online/
https://profarmaceutico.com/Prodotto/acquista-endocet-online/
https://profarmaceutico.com/Prodotto/acquista-eroina-bianca/
https://profarmaceutico.com/Prodotto/acquista-hydrocodone-online/
https://profarmaceutico.com/Prodotto/acquista-instanyl-online/
https://profarmaceutico.com/Prodotto/acquista-l-ritalin-online/
https://profarmaceutico.com/Prodotto/acquista-metadone/
https://profarmaceutico.com/Prodotto/acquista-morfina-solfato/
https://profarmaceutico.com/Prodotto/acquista-opana-online/
https://profarmaceutico.com/Prodotto/acquista-percocet-online/
https://profarmaceutico.com/Prodotto/acquista-phentermine-online/
https://profarmaceutico.com/Prodotto/acquista-stilnox-online/
https://profarmaceutico.com/Prodotto/acquista-suboxone-8mg/
https://profarmaceutico.com/Prodotto/acquista-subutex-online/
https://profarmaceutico.com/Prodotto/acquista-vicodin-online/
https://profarmaceutico.com/Prodotto/acquista-vyvanse-online/
https://profarmaceutico.com/Prodotto/acquista-xanax-2mg/
https://profarmaceutico.com/Prodotto/acquistare-rohypnol-2mg/
https://profarmaceutico.com/Prodotto/acquistare-sibutramina-online/
https://profarmaceutico.com/Prodotto/efedrina-hcl-in-polvere/
https://profarmaceutico.com/Prodotto/ephedrine-hcl-30mg/
https://profarmaceutico.com/Prodotto/sciroppo-di-metadone/
https://profarmaceutico.com/Prodotto/tramadolo-hcl-200mg/
<a href="https://profarmaceutico.com/Prodotto/a-215-ossicodone-actavis/">a-215-ossicodone-actavis</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-adderall-30mg/">acquista-adderall-30mg</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-adipex-online/">acquista-adipex-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-ossicodone-online/">acquista-ossicodone-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-oxycontin-online/">acquista-oxycontin-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-codeina-online/">acquista-codeina-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-adma-online/">acquista-adma-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-ambien/">acquista-ambien</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-ativan-online/">acquista-ativan-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-botox-online/">acquista-botox-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-cerotti-al-fentanil/">acquista-cerotti-al-fentanil</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-codeina-linctus-online/">acquista-codeina-linctus-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-demerol-online/">acquista-demerol-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-depalgo-online/">acquista-depalgo-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-diazepam-online/">acquista-diazepam-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-dilaudid-8mg/">acquista-dilaudid-8mg</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-endocet-online/">acquista-endocet-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-eroina-bianca/">acquista-eroina-bianca</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-green-xanax/">acquista-green-xanax</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-hydrocodone-online/">acquista-hydrocodone-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-instanyl-online/">acquista-instanyl-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-l-ritalin-online/">acquista-l-ritalin-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-metadone/">acquista-metadone</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-morfina-solfato/">acquista-morfina-solfato</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-opana-online/">acquista-opana-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-percocet-online/">acquista-percocet-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-phentermine-online/">acquista-phentermine-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-roxy-roxicodone-30-mg/">acquista-roxy-roxicodone-30-mg</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-stilnox-online/">acquista-stilnox-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-suboxone-8mg/">acquista-suboxone-8mg</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-subutex-online/">acquista-subutex-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-vicodin-online/">acquista-vicodin-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-vyvanse-online/">acquista-vyvanse-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquista-xanax-2mg/">acquista-xanax-2mg</a>;
<a href="https://profarmaceutico.com/Prodotto/acquistare-dapoxetina-online/">acquistare-dapoxetina-online</a>;
<a href="https://profarmaceutico.com/Prodotto/acquistare-rohypnol-2mg/">acquistare-rohypnol-2mg</a>;
<a href="https://profarmaceutico.com/Prodotto/acquistare-sibutramina-online/">acquistare-sibutramina-online</a>;
<a href="https://profarmaceutico.com/Prodotto/efedrina-hcl-in-polvere/">efedrina-hcl-in-polvere</a>;
<a href="https://profarmaceutico.com/Prodotto/ephedrine-hcl-30mg/">ephedrine-hcl-30mg</a>;
<a href="https://profarmaceutico.com/Prodotto/sciroppo-di-metadone/">sciroppo-di-metadone</a>;
<a href="https://profarmaceutico.com/Prodotto/tramadolo-hcl-200mg/">tramadolo-hcl-200mg</a>;
<a href="https://recherchechimique.com/produit/extase-molly/">extase-molly</a>;
<a href="https://recherchechimique.com/produit/bleu-et-jaune-ikea-mdma-220mg/">bleu-et-jaune-ikea-mdma-220mg</a>;
<a href="https://recherchechimique.com/produit/acheter-vyvanse-en-ligne/">cheter-vyvanse-en-ligne</a>;
<a href="https://recherchechimique.com/produit/5-meo-dmt-5ml-purecybine/">5-meo-dmt-5ml-purecybine</a>;
<a href="https://recherchechimique.com/produit/a-champignons-magiques/">a-champignons-magiques</a>;
<a href="https://recherchechimique.com/produit/acheter-du-marbre-hash-en-ligne/">acheter-du-marbre-hash-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-3-meo-pcp-en-ligne/">acheter-3-meo-pcp-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-acquista-xanax-2mg-en-ligne/">acheter-acquista-xanax-2mg-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-de-lheroine-en-ligne/">acheter-de-lheroine-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-adderall-xr-en-ligne/">acheter-adderall-xr-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-de-la-codeine-en-ligne/">acheter-de-la-codeine-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-de-la-methadone-en-ligne/">acheter-de-la-methadone-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-de-la-morphine-en-ligne/">acheter-de-la-morphine-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-des-champignons-magiques-alacabenzi-en-ligne/">acheter-des-champignons-magiques-alacabenzi-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-des-champignons-magiques-albino-a-en-ligne/">acheter-des-champignons-magiques-albino-a-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-des-champignons-magiques-albino-penis-envy-en-ligne/">acheter-des-champignons-magiques-albino-penis-envy-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-deschampignons-magiques-albinos-cambodgiens-en-ligne/">acheter-deschampignons-magiques-albinos-cambodgiens-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-du-cristal-de-mdma-en-ligne/">acheter-du-cristal-de-mdma-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-hydrocodone-en-ligne/">acheter-hydrocodone-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-oxycontin-en-ligne/">acheter-oxycontin-en-ligne</a>;
<a href="https://recherchechimique.com/produit/acheter-percocet-en-ligne/">acheter-percocet-en-ligne</a>;
<a href="https://recherchechimique.com/produit/ayahuasca-dmt/">ayahuasca-dmt</a>;
<a href="https://recherchechimique.com/produit/bonbons-au-lsd/">bonbons-au-lsd</a>;
<a href="https://recherchechimique.com/produit/brun-donkey-kong-mdma-260mg/">brun-donkey-kong-mdma-260mg</a>;
<a href="https://recherchechimique.com/produit/buvards-lsd/">buvards-lsd</a>;
<a href="https://recherchechimique.com/produit/champignons-magiques-albino-treasure-coast/">champignons-magiques-albino-treasure-coast</a>;
<a href="https://recherchechimique.com/produit/comprimes-de-gel-de-lsd/">comprimes-de-gel-de-lsd</a>;
<a href="https://recherchechimique.com/produit/cristaux-de-ketamine/">cristaux-de-ketamine</a>;
<a href="https://recherchechimique.com/produit/deadhead-chemist-dmt-3-cartouches-deal-5ml/">deadhead-chemist-dmt-3-cartouches-deal-5ml</a>;
<a href="https://recherchechimique.com/produit/deadhead-chimiste-5-meo-dmt-cartouche-5ml/">deadhead-chimiste-5-meo-dmt-cartouche-5ml</a>;
<a href="https://recherchechimique.com/produit/deadhead-chimiste-dmt/">deadhead-chimiste-dmt</a>;
<a href="https://recherchechimique.com/produit/glace-methamphetamine/">glace-methamphetamine</a>;
<a href="https://recherchechimique.com/produit/ketamine-hcl/">ketamine-hcl</a>;
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/a-215-ossicodone-actavis/" rel="dofollow">a-215-ossicodone-actavis</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-adderall-30mg/" rel="dofollow">acquista-adderall-30mg</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-adipex-online/" rel="dofollow">acquista-adipex-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-ossicodone-online/" rel="dofollow">acquista-ossicodone-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-oxycontin-online/" rel="dofollow">acquista-oxycontin-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-codeina-online/" rel="dofollow">acquista-codeina-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-adma-online/" rel="dofollow">acquista-adma-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-ambien/" rel="dofollow">acquista-ambien</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-ativan-online/" rel="dofollow">acquista-ativan-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-botox-online/" rel="dofollow">acquista-botox-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-cerotti-al-fentanil/" rel="dofollow">acquista-cerotti-al-fentanil</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-codeina-linctus-online/" rel="dofollow">acquista-codeina-linctus-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-demerol-online/" rel="dofollow">acquista-demerol-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-depalgo-online/" rel="dofollow">acquista-depalgo-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-diazepam-online/" rel="dofollow">acquista-diazepam-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-dilaudid-8mg/" rel="dofollow">acquista-dilaudid-8mg</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-endocet-online/" rel="dofollow">acquista-endocet-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-eroina-bianca/" rel="dofollow">acquista-eroina-bianca</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-green-xanax/" rel="dofollow">acquista-green-xanax</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-hydrocodone-online/" rel="dofollow">acquista-hydrocodone-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-instanyl-online/" rel="dofollow">acquista-instanyl-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-l-ritalin-online/" rel="dofollow">acquista-l-ritalin-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-metadone/" rel="dofollow">acquista-metadone</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-morfina-solfato/" rel="dofollow">acquista-morfina-solfato</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-opana-online/" rel="dofollow">acquista-opana-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-percocet-online/" rel="dofollow">acquista-percocet-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-phentermine-online/" rel="dofollow">acquista-phentermine-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-roxy-roxicodone-30-mg/" rel="dofollow">acquista-roxy-roxicodone-30-mg</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-stilnox-online/" rel="dofollow">acquista-stilnox-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-suboxone-8mg/" rel="dofollow">acquista-suboxone-8mg</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-subutex-online/" rel="dofollow">acquista-subutex-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-vicodin-online/" rel="dofollow">acquista-vicodin-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-vyvanse-online/" rel="dofollow">acquista-vyvanse-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-xanax-2mg/" rel="dofollow">acquista-xanax-2mg</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquistare-dapoxetina-online/" rel="dofollow">acquistare-dapoxetina-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquistare-rohypnol-2mg/" rel="dofollow">acquistare-rohypnol-2mg</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquistare-sibutramina-online/" rel="dofollow">acquistare-sibutramina-online</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/efedrina-hcl-in-polvere/" rel="dofollow">efedrina-hcl-in-polvere</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/ephedrine-hcl-30mg/" rel="dofollow">ephedrine-hcl-30mg</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/sciroppo-di-metadone/" rel="dofollow">sciroppo-di-metadone</a>
<a href="https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/tramadolo-hcl-200mg/" rel="dofollow">tramadolo-hcl-200mg</a>
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/a-215-ossicodone-actavis/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-adderall-30mg/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-adipex-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-ossicodone-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-oxycontin-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-codeina-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-adma-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-ambien/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-ativan-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-botox-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-cerotti-al-fentanil/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-codeina-linctus-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-demerol-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-diazepam-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-dilaudid-8mg/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-endocet-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-eroina-bianca/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-green-xanax/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-hydrocodone-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-instanyl-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-l-ritalin-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-metadone/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-morfina-solfato/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-opana-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-percocet-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-phentermine-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-roxy-roxicodone-30-mg/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-stilnox-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-suboxone-8mg/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-subutex-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-vicodin-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-vyvanse-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquista-xanax-2mg/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquistare-dapoxetina-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquistare-rohypnol-2mg/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/acquistare-sibutramina-online/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/efedrina-hcl-in-polvere/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/ephedrine-hcl-30mg/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/sciroppo-di-metadone/
https://www.google.it/url?q=https://profarmaceutico.com/Prodotto/tramadolo-hcl-200mg/