 Your new post is loading...

|
Scooped by
mhryu@live.com
Today, 2:48 PM
|
Canonical nucleic acids (DNA and RNA) naturally store genetic information with high density and programmability, making them promising candidates for molecular data storage. However, their susceptibility to degradation under harsh conditions, such as extreme pH, nuclease activity, and chemical attack, limits practical applications. In contrast, non-canonical nucleic acids (ncNAs) with natural or synthetic structural modifications exhibit enhanced stability and unique functional potential. This review systematically summarizes the fundamental properties of ncNAs, evaluates their suitability for molecular data storage, and discusses how their distinctive advantages may overcome the intrinsic limitations of canonical nucleic acids while addressing challenges in next-generation storage systems. Canonical nucleic acids, with high storage density, are promising media for data storage but are limited by instability. Here, authors summarise the unique properties and advantages of non-canonical nucleic acids in data storage and highlight key challenges for their practical application.

|
Scooped by
mhryu@live.com
Today, 2:33 PM
|
Nodule-forming bacteria play crucial roles in plant health and nutrition by providing fixed nitrogen to leguminous plants. Despite the importance of this relationship, how nodule-forming bacteria are affected by plant exudates and soil minerals is not fully characterized. Here, the effects of plant-derived methanol and lanthanide metals on the growth of nitrogen-fixing Rhizobiales are examined. Prior work has demonstrated that select Bradyrhizobium strains can assimilate methanol only in the presence of lanthanide metals; however, the pathway enabling assimilation remains unknown. In this study, we characterize Bradyrhizobium diazoefficiens USDA 110, Bradyrhizobium sp. USDA 3456, and Sinorhizobium meliloti 2011 to determine the pathways involved in methanol metabolism in previously characterized strains, other clades of Bradyrhizobium, and the more distantly related Sinorhizobium. Based on genomic analyses, we hypothesized that methanol assimilation in these organisms occurs via the lanthanide-dependent methanol dehydrogenase XoxF, followed by oxidation of formaldehyde via the glutathione-linked oxidation pathway, subsequent oxidation of formate via formate dehydrogenases, and finally assimilation of CO2 via the Calvin-Benson-Bassham (CBB) cycle. Transcriptomics revealed upregulation of the aforementioned pathways in USDA 3456 during growth with methanol. Enzymatic assays demonstrated increased activity of the glutathione-linked oxidation pathway and formate dehydrogenases in all strains during growth with methanol compared to succinate. 13C-labeling studies confirmed the presence of CBB intermediates and label incorporation during growth with methanol. Our findings provide multiple lines of evidence supporting the proposed XoxF-CBB pathway and, combined with genomic analyses, suggest that this metabolism is widespread among Bradyrhizobium and Sinorhizobium species.

|
Scooped by
mhryu@live.com
Today, 1:59 PM
|
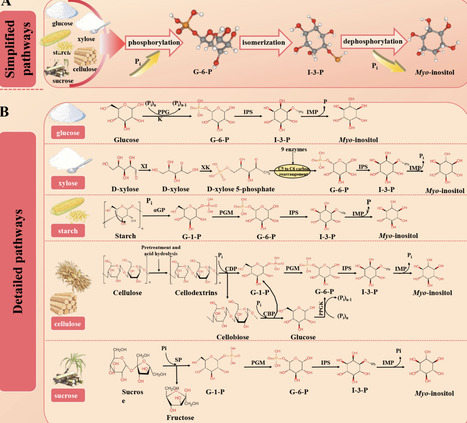
Myo-inositol, a high-value cyclic polyol, is increasingly sought by pharmaceutical, food, feed, and cosmetic industries. This review systematically surveys the latest biotechnological advances poised to replace traditional, high-pollution methods. First, multienzyme cascades that convert renewable carbohydrates─starch, glucose, xylose, cellulose, sucrose─are reviewed, highlighting immobilized reactors, porous microspheres, biomimetic mineralized capsules, and biofilm systems that boost stability, reusability, and space-time yields. Second, microbial cell-factory strategies are examined, covering chassis benchmarking (E. coli, Pichia pastoris, Kluyveromyces marxianus, cyanobacteria), carbon-flux redirection, glucose-glycerol synergistic feeding, and dynamic regulatory circuits. A unified analysis pinpoints recurrent bottlenecks─cofactor imbalance, enzyme thermostability gaps, narrow substrate spectra, product inhibition, and downstream complexity─and distills the targeted fixes discussed in the field, from cofactor regeneration circuits to modular process design. By integrating cutting-edge research with industrial techno-economic indicators, this review offers a comprehensive roadmap for sustainable, cost-competitive myo-inositol biomanufacturing and guides future research toward greener production systems.

|
Scooped by
mhryu@live.com
Today, 1:50 PM
|
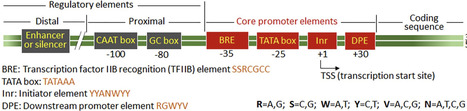
Plant-based expression systems, known as molecular farming, have emerged as sustainable and scalable platforms for producing recombinant proteins used in pharmaceuticals, industrial enzymes, and agricultural products. Among the key determinants of transgene performance, promoter elements play a central role in defining transcriptional strength, specificity, and regulation. This review highlights current advances in promoter engineering tailored for plant systems, encompassing natural, synthetic, hybrid, inducible, and tissue-specific promoters used in stable transgenic plants, transient expression systems, and plant cell cultures. The structural and functional features of promoter elements are discussed, along with strategies to mitigate challenges such as transcriptional silencing, genomic context dependency, and variability cross species and production platform. Emerging synthetic biology tools, such as CRISPR-based transcriptional control, high-throughput screening, and machine learning–assisted promoter design, are enabling the creation of tunable, orthogonal promoters suited for complex multigene expression. As promoter engineering continues to evolve, it remains foundational to advancing plant molecular farming and expanding the role of plants as versatile biofactories for high-value recombinant proteins.

|
Scooped by
mhryu@live.com
Today, 1:30 PM
|
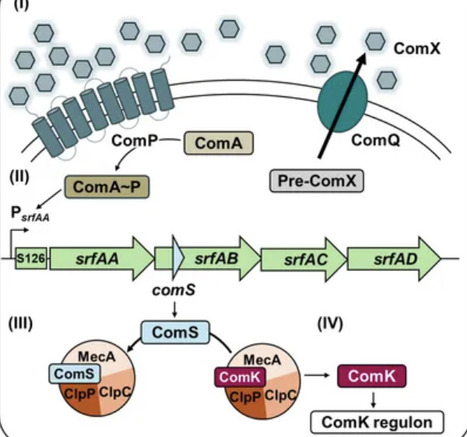
In its natural soil habitat, B. subtilis regularly encounters fluctuating conditions that require adaptive survival strategies, including the production and secretion of antimicrobial compounds. One such compound, surfactin, play a central role in multicellular differentiation processes such as biofilm formation, swarming, and competence development. Competence and surfactin biosynthesis are transcriptionally co-regulated via the quorum sensing-mediated activation of the srfAABCD operon, which contains comS in a distinct ORF overlapping with srfAB. This study aimed at uncoupling competence from surfactin production by introducing targeted stop mutations in comS to selectively disrupt competence without affecting surfactin synthesis. For this, we introduced single nucleotide polymorphisms (SNPs) that preserved the srfAB codons, while simultaneously introducing a premature stop codon in comS. The effects on competence development were assessed using luciferase-based reporter assays monitoring the ComS-dependent expression of comK and comGA expression. Surfactin production was analyzed by mass spectrometry imaging and phenotypic assays examining the impact on multicellular behavior. Our findings demonstrate that the generated point mutations severely reduce competence gene expression, measured via PcomKand PcomGAactivity, to levels comparable with a full comS deletion, while leaving multicellular behaviors such as biofilm and pellicle formation, as well as swarming and sliding motility, unaffected. Thus, ComS is specifically essential for competence development but dispensable for other surfactin-mediated multicellular processes and not involved in structuring biofilms. Taken together, our results demonstrate that it is possible to genetically decouple competence from other developmental pathways in B. subtilis.

|
Scooped by
mhryu@live.com
Today, 1:21 PM
|
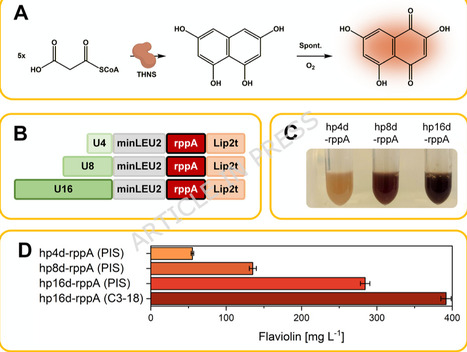
Yarrowia lipolytica is an emerging host for producing acetyl-CoA– and malonyl-CoA–derived chemicals. However, most processes rely on yeast nitrogen base (YNB), a historical formulation with poorly controlled trace metal content. This variability impairs metabolic performance, limits reproducibility, and complicates process transfer. Commercial YNB batches differed markedly, causing 1.5–2-fold variation in growth and docosahexaenoic acid (DHA) production. We developed a malonyl-CoA–responsive flaviolin reporter strain and combined it with a structured Design of Experiments (DoE) workflow to systematically re-engineer YNB mineral composition. Dissection of all 20 YNB components revealed that vitamins are dispensable under the tested conditions, whereas a small subset of salts and trace elements - particularly ZnSO4, FeCl3, KH2PO4, MgSO4, CaCl2, and CuSO4 - dominantly shape precursor availability and product formation. One-factor-at-a-time (OFAT), factorial, steepest ascent, and central composite designs converged in an optimized synthetic mineral medium assembled entirely from individual salts and trace metals. This formulation increased flaviolin titers to 1.41 ± 0.08 g L-1, a more-than threefold improvement over commercial YNB, while ensuring high reproducibility. Key mineral interventions also translated to complex pathways: omission of ZnSO4 increased PUFA titers by 7.6-fold (docosapentaenoic acid, DPA) and 58-fold (eicosapentaenoic acid, EPA) and enhanced DHA formation in independent production strains. The defined formulation substantially reduces cost and eliminates batch-to-batch variability inherent to commercial YNB powders. Our results establish mineral balancing as a major yet underused lever for improving acetyl-CoA– and malonyl-CoA–derived production in Y. lipolytica and demonstrate a generalizable, model-guided workflow for creating simplified, reproducible, and cost-efficient synthetic media for non-conventional yeast cell factories.

|
Scooped by
mhryu@live.com
Today, 1:00 PM
|
Numerous Gram-negative bacteria possess N-acyl-L-homoserine lactone (AHL)-mediated quorum-sensing (QS) systems that regulate the activation of specific genes. Burkholderia plantarii causes rice seedling blight by producing the phytotoxin tropolone. In this study, we investigated multiple AHL-type QS systems in B. plantarii MAFF 301723T and their involvement in virulence regulation. MAFF 301723 harbors three AHL-mediated QS systems, designated plaI1/plaR1, plaI2/plaR2, and plaI3/plaR3. The plaI1/plaR1 system, which produces N-octanoyl-l-homoserine lactone, is functional and essential for swarming motility. When forced expression of plaI2 induces the biosynthesis of 3-OH-C10-HSL, it was suggested that expression is rarely observed in wild-type MAFF 301723. The plaI3 gene directs the synthesis of the putative C16:2-HSL, which is a rare AHL bearing two double bonds in the hexadecanoyl chain that has not been previously reported in Burkholderia spp. The plaI3/plaR3-QS system is crucial for tropolone production. These findings suggest that multiple QS systems collectively contribute to the complex virulence regulation of B. plantarii, thereby providing new insights into the development of QS-targeted biocontrol strategies for agriculture.

|
Scooped by
mhryu@live.com
Today, 12:55 PM
|
Photoreceptor proteins regulate fundamental biological processes such as vision, photosynthesis and circadian rhythms. A large photoreceptor subfamily uses vitamin B12 derivatives for light sensing, contrasting with the well-established mode of action of these organometallic derivatives in thermally activated enzymatic reactions. The exact molecular mechanism of B12 photoreception and how this differs from the thermal pathways remains unknown. Here we provide a detailed description of photoactivation in the prototypical B12 photoreceptor CarH from nanoseconds to seconds, combining time-resolved and temperature-resolved structural and spectroscopic methods with quantum chemical calculations. Building on the crystal structures of the initial tetrameric dark and final monomeric light-activated states, our structural snapshots of key intermediates in the truncated B12-binding domain illustrate how photocleavage of a cobalt–carbon (Co–C) bond within the B12 chromophore adenosylcobalamin triggers a series of structural changes that propagate throughout CarH. Breakage of the photolabile Co–C5′ bond leads to the formation of a previously unknown adduct that links the C4′ position of the adenosyl moiety to the Co ion and can subsequently be cleaved thermally over longer timescales to allow release of the adenosyl group, ultimately causing tetramer dissociation. This adduct, which differentiates CarH from thermally activated B12 enzymes, steers the photoactivation pathway and acts as the molecular bridge between photochemical and photobiological timescales. The biological relevance of our study is corroborated by kinetic data on full-length CarH in the presence of DNA. Our results offer a spatiotemporal understanding of CarH photoactivation and pave the way for designing B12-dependent photoreceptors for optogenetic applications. Spatiotemporal insight into photoactivation of the prototypical B12 photoreceptor CarH is revealed across nine orders of magnitude in time, identifying a transient adduct that distinguishes it from thermally activated B12 enzymes.

|
Scooped by
mhryu@live.com
Today, 12:32 PM
|
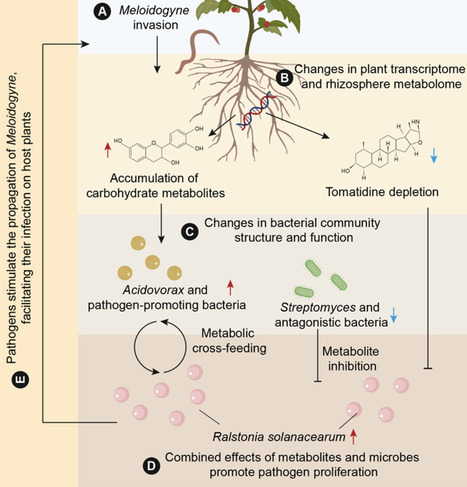
Root-knot nematodes cause substantial crop losses by compromising plant immunity and facilitating invasion by soil-borne bacterial pathogens, yet the mechanisms underlying nematode-facilitated co-infection remain poorly understood. Here, we quantify the global prevalence of nematode-pathogen co-infection and integrate multi-omic analyses across greenhouse and in vitro experiments. We show that nematode invasion activates plant defense gene expression but concurrently disrupts rhizosphere homeostasis, resulting in microbiome dysbiosis that overrides host resistance. Meloidogyne invasion induces pronounced metabolic reprogramming, characterized by depletion of tomatidine and accumulation of carbohydrate metabolites such as galactose. These shifts selectively suppress Streptomyces-dominated antagonistic microbiota while enriching Acidovorax, which exhibits nutritional synergy with Ralstonia. Using synthetic microbial community transplantation, we demonstrate a functional transition from pathogen-suppressive to pathogen-permissive bacteriomes following nematode invasion. Together, our findings reveal how nematodes and bacterial pathogens cooperatively subvert plant-microbe metabolic signaling to undermine rhizosphere immunity, highlighting actionable targets for microbiome-based disease control.

|
Scooped by
mhryu@live.com
Today, 12:15 AM
|
Mass spectrometry-based proteomics offers a powerful tool for characterizing enzyme expression in engineered strains, yet rapid generation of large strain libraries creates proteomic analysis bottlenecks. The critical limitation lies in manual sample preparation—protein extraction, denaturation, reduction, desalting, and digestion—which is time-consuming and risks compromising reproducibility. To overcome this bottleneck, we developed a novel “strain-to-peptide conversion” (SPC) strategy for high-throughput proteome profiling in microbial cell factories. This automated workflow integrates bacterial lysis, magnetic solid-phase alkylation (mSPA)-based protein enrichment, contaminant removal, and rapid digestion through a commercial liquid handling system, processing 96 samples within 1 hour. Compared to the well-established single-pot solid-phase-enhanced sample preparation (SP3) method, SPC achieves a 94% reduction in processing time while maintaining equivalent protein identification depth. Furthermore, the quantification of membrane proteins was increased by 28%. Meanwhile, the method demonstrated exceptional reproducibility, with intra- and inter-batch Pearson correlation coefficients exceeding 0.95. Leveraging this platform, we processed 96 E. coli samples simultaneously, with reliable quantitative data revealing significant regulation of proteins primarily associated with translation, transmembrane transport, and metabolic processes following overexpression of key tricarboxylic acid (TCA) cycle enzymes. These results establish the SPC strategy as an efficient high-throughput solution for large-scale strain proteome analysis, advancing rational cell factory design in metabolic engineering and synthetic biology.

|
Scooped by
mhryu@live.com
Today, 12:04 AM
|
Protein aggregation plays a central role in the pathogenesis of many neurodegenerative diseases and poses major challenges in protein engineering. A key driver of this process is the presence of aggregation-prone regions (APRs) within protein sequences. We present AggrescanAI, a deep learning-based tool that predicts residue-level aggregation propensity directly from sequence. It leverages contextual embeddings from the ProtT5 protein language model, which captures rich information implicitly encoded in the sequence, without requiring structural data. The model was trained on a set of experimentally annotated APRs, expanded via homology transfering, evaluated by cross-validation, and validated with an external benchmark. AggrescanAI outperforms state of the art predictors and captures aggregation shifts induced by pathogenic mutations. To facilitate accessibility, we provide a user-friendly and fully open Google Colab notebook: https://gitlab.com/bioinformatics-fil/aggrescanai. AggrescanAI represents a new generation of sequence-based aggregation predictors, powered by deep learning and protein language models.

|
Scooped by
mhryu@live.com
February 3, 11:52 PM
|
Branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, are essential nutrients for animals that must be obtained from the diet, as mammals cannot synthesize them. This study developed an engineered Pichia pastoris for efficient production of BCAAs-enriched single-cell protein (SCP) through synergistic integration of metabolic engineering and artificial intelligence. We first enhanced BCAAs biosynthesis by overexpressing the Ilv3 gene (encoding dihydroxy-acid dehydratase) via CRISPR-Cas9. Subsequently, the PMPEPE (P. pastoris Mutation Predictor for Enhanced Protein Expression) model screened endogenous proteins with high BCAAs content, enabling AI-driven in silico design of a BCAAs-rich variant, M0504 (35% BCAAs composition). This engineered protein was successfully expressed in P. pastoris. High-cell-density fermentation demonstrated that the engineered strain HTX33-ILV3-M0504 exhibited significantly increased crude protein content during methanol induction and superior biomass accumulation compared to controls. The BCAAs content in SCP reached 9.8 mg/100 mg (equivalent to 11.9 g/L in the fermentation broth), representing a 37.1% increase over the wild-type strain HTX-33 (7.2 mg/100 mg). Transcriptomics analysis revealed that Ilv3 overexpression upregulated key genes in the BCAAs synthesis pathway while modulating metabolic homeostasis through the TCA cycle, methanol assimilation, and carbon–nitrogen co-utilization. This work establishes a scalable strategy for industrial production of functional SCP enriched with BCAAs.

|
Scooped by
mhryu@live.com
February 3, 11:10 PM
|
Bacterial microcompartments (BMC) are protein-based organelles that spatially organise metabolic pathways in prokaryotes, playing critical roles in enhancing metabolic processes and microbe fitness. Notably, many bacterial species possess multiple types of BMCs. While recent studies have advanced our knowledge about the assembly and function of individual BMC types, the mechanisms governing the coexistence and interplay of distinct BMC families within a single bacterial cell remain poorly understood. Here, we engineered Salmonella enterica serovar Typhimurium LT2 to co-express native 1,2-propanediol utilisation (Pdu) BMCs and synthetic α-carboxysomes (α-CBs), providing a unique platform for dissecting their assembly dynamics and functional crosstalk. By exploiting super-resolution fluorescence imaging, electron microscopy, biochemical and enzymatic assays, our studies demonstrate the formation of hybrid BMCs through the exchange of shell proteins between Pdu BMCs and α-CBs, whereas cargo proteins exhibit only limited compatibility, highlighting the specificity of encapsulation mechanisms. Furthermore, the generated hybrid BMCs display altered mobility and enzymatic activities, revealing emergent properties arising from shell protein interchangeability. Our findings provide insights into the inherent structural plasticity and modular architecture of BMCs. More broadly, this study has implications for deciphering how bacterial cells modulate the construction and functions of diverse metabolic modules within a single cellular context and could inform the rational design and engineering of synthetic organelles and bio-factories with tailored metabolic functions for biotechnological applications.
|

|
Scooped by
mhryu@live.com
Today, 2:38 PM
|
The human gastrointestinal tract is home to a diverse community of microorganisms from all domains of life, collectively referred to as the gut microbiome. While gut bacteria have been studied extensively in relation to human host health and physiology, other constituents remain underexplored. This includes the gut virome, the collection of bacteriophages, eukaryotic viruses, and other mobile genetic elements present in the intestine. Like gut bacteria, the gut virome has been causatively linked to human health and disease. However, the gut virome is substantially more difficult to characterize, given its high diversity and complexity, as well as multiple challenges related to in vitro cultivation and in silico detection and annotation. In this mini-review, we describe various methodologies for examining the gut virome using both culture-dependent and culture-independent tools. We highlight in vitro and in vivo approaches to cultivate viruses and characterize viral-bacterial host dynamics, as well as high-throughput screens to interrogate these relationships. We also outline a general workflow for identifying and characterizing uncultivated viral genomes from fecal metagenomes, along with several key considerations throughout the process. More broadly, we aim to highlight the opportunities to synergize and streamline wet- and dry-lab techniques to robustly and comprehensively interrogate the human gut virome.

|
Scooped by
mhryu@live.com
Today, 2:02 PM
|
Methanotrophs are of fundamental importance in the global methane cycle and hold promise for the bioconversion of methane into valuable products. This study elucidates the impact of alternating aerobic–anoxic conditions on metabolism of the two phylogenetically distinct groups of methanotrophs: Methylomonas koyamae (type I, γ-proteobacteria) and Methylocystis bryophila (type II, α-proteobacteria). Using batch cultivations with single and repeated oxygen pulses, we demonstrated that cyclic aerobic–anoxic alternation significantly increased the secretion of organic compounds. Notably, acetate secretion by the type II methanotroph M. bryophila was observed for the first time. Genome-scale metabolic modeling and flux balance analysis revealed that acetate secretion serves as an auxiliary ATP-generating pathway for M. bryophila under oxygen limitation. We further uncovered a dynamic mechanism: aerobic phases trigger methane uptake and build up intracellular organic carbon pools, while anoxic phases redirect these pools toward acetate and/or hydrogen release to sustain survival under oxygen limitation. This cyclic regimen effectively channels more methane-derived carbon into extracellular acetate. Our findings underscore that redox conditions critically shape methanotrophic metabolism, influencing both the biological methane cycle and the global climate.

|
Scooped by
mhryu@live.com
Today, 1:57 PM
|
The research on synthetic methylotrophic bacteria for one-carbon (C1) feedstock assimilation has garnered substantial interest and is regarded as the forefront of biomanufacturing advancements. Nevertheless, the effective utilization of C1 feedstocks faces challenges due to inadequate tolerance toward C1 compounds. This study elucidates that the buildup of formaldehyde causes severe DNA–protein cross-linking (DPC), and thus hampers growth and methanol assimilation in E. coli. To tackle this issue, we exploited a metalloproteinase, SpWss1, from Schizosaccharomyces pombe. By fine-overexpressing SpWss1 in the E. coli genome, we were able to alleviate DPC damage and enhance formaldehyde tolerance. Remarkably, the engineered strain displayed a 10-fold increase in the amount of methanol assimilated (142 mM) compared to that of the control strain lacking SpWss1 (14 mM). Moreover, through iterative substrate feeding of methanol and xylose in shake-flask experiments, the genetically modified strain exhibited improved consumption levels, reaching up to 309 mM (∼10 g/L), making it one of the highest methanol-consuming strains among all E. coli strains without adaptive evolution. Additionally, the modified strain significantly enhanced the sustainable production of valuable products, such as triacetic acid lactone and fatty acids, from methanol. Overall, our findings underscore the significant scientific and biotechnological importance of addressing DPC to optimize C1 assimilation, providing valuable insights for sustainable chemistry, engineering, and industrial biotechnology applications.

|
Scooped by
mhryu@live.com
Today, 1:45 PM
|
Mushroom-forming basidiomycetes are increasingly recognized for their significant potential to remediate polluted environments and mitigate climate change. This review synthesizes evidence positioning mushroom-forming basidiomycetes at the nexus of ecological resilience and a sustainable bioeconomy, highlighting their dual roles in environmental repair and green innovation. Ectomycorrhizal (ECM species) enhance carbon acquisition by plants and long-term soil carbon sequestration; ECM-dominant forests stockpile upto 70% more below-ground carbon than their non-mycorrhizal counterparts. Saprotrophic fungi drive lignocellulose degradation, nutrient cycling, and the stabilization of soil organic matter. Basidiomycetes also play a crucial role in mycoremediation by degrading recalcitrant contaminants (pesticides, hydrocarbons) and immobilizing heavy metals. Furthermore, mycelium-based biomaterials are being developed as green-technology alternatives to plastics and synthetic foams, reflecting the growing commercialization of fungal biotechnology, as evidenced by the global mycelium material industry projected to exceed USD 5 billion by 2032. The intersection of ecological function and economic value positions mushrooms at the forefront of the circular bioeconomy. However, challenges remain, including production scalability, environmental sensitivity, and economic viability. Addressing these challenges through interdisciplinary research could unlock the full potential of fungi as nature-based climate solutions.

|
Scooped by
mhryu@live.com
Today, 1:24 PM
|
Komagataella pastoris is extensively used as a microbial cell factory for the production of recombinant proteins and high-value compounds. However, tightly controlled promoter systems responsive to safe and economical inducers are required for precise metabolic and pathway engineering in this yeast species. Cumate-inducible promoters are an ideal choice due to the safety and low cost of cumate. In this study, we systematically optimised the insertion sites of the CuO operator sequence within the strong promoter PGCW14 to isolate a high-activity variant that we designated as PGCWCuO03. To fine-tune the expression of the repressor protein CymR, we developed a truncated promoter of PGAP, designated as PGAP200. Based on the optimal promoter PGCWCuO03 and the CymR expression unit, we constructed a robust CymR/CuO-mediated cumate-inducible promoter, designated as Pgc, in K. pastoris. Pgc demonstrated outstanding induction properties, resulting in an approximately 11-fold increase in target protein production following induction. Promoter substitution assays validated the effectiveness of Pgc in temporal gene expression control, highlighting the significant potential of this promoter for both basic research and industrial bioprocessing applications in synthetic biology and biotechnology in K. pastoris.

|
Scooped by
mhryu@live.com
Today, 1:16 PM
|
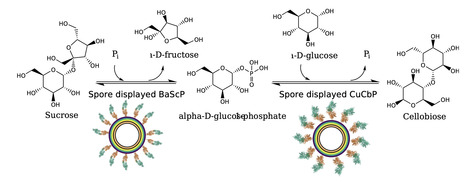
Cellobiose (4-O-β-D-Glucopyranosyl-D-glucopyranose) is an important disaccharide utilized, for example in food and cosmetics. It can be enzymatically synthesized involving two steps from sucrose and glucose, where first the sucrose undergoes phosphorolysis by sucrose phosphorylase, yielding glucose 1-phosphate and fructose. Glucose 1-phosphate is then combined with glucose into cellobiose, releasing phosphate in a reaction catalyzed by cellobiose phosphorylase. To better control and reuse the enzymes in the two main reaction steps, immobilization on Bacillus subtilis spores is a promising approach due to the ease of production and recyclability. Here we describe the display of a sucrose phosphorylase and a cellobiose phosphorylase on B. subtilis spores through fusion with the crust protein CotY, to our knowledge, marking the first use of multiple enzymes directly displayed on the spore surface during sporulation in a reaction cascade. While immobilization had no effect on thermostability, we demonstrate the recyclability of the individual spore variants over four reaction cycles at 45 °C with sucrose phosphorylase maintaining 35% of its initial activity and cellobiose phosphorylase maintaining 65%. Both spore variants were used together to catalyse a reaction cascade in a separated two-pot, as well as in a one-pot reaction. The one-pot reaction achieved a 90% yield with respect to the initially available 40 mM of glucose. The one-pot cascade maintained activity after being recycled five times over the course of 120 hours. Furthermore, we report on improving the reaction yield in the two-pot reaction from 60% to 80% by using calcium to precipitate excess phosphate. In this study we demonstrate that spores are a suitable immobilization platform for multistage reaction cascades. The spores displaying biocatalysts can be recovered and reused over multiple reaction cycles. The immobilization of glycosylic enzymes on spores enables cost-effective, scalable enzyme production on a temperature-resistant carrier that facilitates purification. The potential modularity of this approach adds to the adaptability of the system to different requirements in terms of substrate and product.

|
Scooped by
mhryu@live.com
Today, 12:57 PM
|
Crops depend on microbial partners for their growth, development, and overall resilience. A pivotal understanding has emerged showing the direct involvement of the root microbiota in regulating the tiller number of rice, a crucial architecture that influences yield. Novel frontiers in microbiological applications for agriculture highlight the profound role of the root microbiota in shaping crop architecture to boost productivity. We propose that improvements in crop production are moving from a genetic perspective on “architecture” to embracing “holobiont architecture.” As such, microbial orchestration provides a dynamic fine-tune function for breeding “architecture-smart crops” characterised by phenotypic plasticity under environmental uncertainty.

|
Scooped by
mhryu@live.com
Today, 12:44 PM
|
Bacteria use diverse mechanisms to protect themselves against phages. Many antiphage systems form large oligomeric complexes, but how oligomerization is regulated during phage infection remains mostly unknown. Here we demonstrate that the bacterial immunity protein ring-activated zinc-finger RNase (RAZR) assembles into an active, 24-meric ring around the circumference of large ring structures formed by two unrelated phage proteins: a putative recombinase and a portal protein. Each multi-layered, megadalton-scale complex enables RAZR to cleave RNA nonspecifically to inhibit translation and restrict phage propagation. The recognition of unrelated phage proteins that form rings with similar diameters indicates that these proteins not only bind to RAZR but also enforce a geometry crucial to activation. The lack of large ring structures in the host probably prevents auto-immunity and RAZR activation before infection. The infection-triggered oligomerization of RAZR mirrors pathogen-induced oligomerization in eukaryotic innate immune complexes, underscoring a common principle of immunity across biology. An antiphage defence system has an activation mechanism that relies on the sensing of phage-encoded proteins that enforce geometry crucial to activation and are not typically present in non-infected cells.

|
Scooped by
mhryu@live.com
Today, 12:28 PM
|
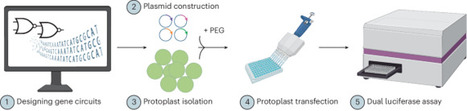
Synthetic gene circuits are powerful tools for precisely programming gene expression and introducing novel cellular functions. However, their development and application in plants has lagged behind other systems, due mainly to the limited availability of modular genetic parts. We recently developed a CRISPR interference (CRISPRi)-based synthetic gene circuit system for programming gene expression in plants. Using a robust and high-throughput protoplast-based dual luciferase assay, we demonstrated the development, testing and functionality of these circuits in various plant species. Here we detail the key design principles and considerations for building and testing programmable and reversible CRISPRi-based gene circuits in plants. We also provide detailed procedures for isolating protoplasts from multiple plant species, including Arabidopsis thaliana, Brassica napus, Triticum aestivum and Physcomitrium patens. Furthermore, we provide step-by-step instructions for the 96-well plate-based protoplast transfection assay for testing genetic parts and synthetic circuits, using a dual luciferase assay. The detailed descriptions of these developed systems will enhance the efficiency and reproducibility of the construction, testing, and implementation of synthetic gene circuits in a variety of plant species. This protocol enables the design and testing of CRISPRi-based gene circuits in plants within ~4 weeks. This protocol details the design principles and procedures for building programmable CRISPRi-based gene circuits, and for testing individual components and the assembled circuits in various plant species using a high-throughput protoplast-based assay.

|
Scooped by
mhryu@live.com
Today, 12:09 AM
|
Genome minimization, including the deletion of endogenous gene clusters that encode natural products, is a common strategy to improve the yield of heterologous products. We have been interested in developing Burkholderia sp. FERM BP-3421 as an alternative bacterial host. Instead of indiscriminately deleting gene clusters, which may have deleterious effects, we guided our efforts using transcriptomics data from production cultures. The genome of FERM BP-3421 is subdivided into two chromosomes and two plasmids. The top transcribed gene clusters were those encoding polyketide-nonribosomal peptide spliceostatins on plasmid p1 and nonribosomal peptide selethramide on chromosome 1. Deletion of the spliceostatin cluster had been shown to improve titers of the ribosomal peptide capistruin, whereas we showed that deletion of the selethramide cluster had no effect on capistruin titers. We next targeted the two endogenous plasmids using a CRISPR-Cas12a strategy, resulting in an 11 % reduction in genome size. The plasmid cured strains showed improved growth and 20–40 % increased production of capistruin depending on whether one or both plasmids were deleted. However, deletion of p2 alone negatively affected the heterologous production of two distinct polyketide-nonribosomal peptides. The p2− strain produced only 5–23 % of the glidobactin A and megapolipeptin A titers compared to the wild type, respectively, whereas titers were restored to wild type levels in the p1− p2− strain. The observation that p2 appears to contain functions that support polyketide-nonribosomal peptide biosynthesis was unexpected and sets the stage for future studies aimed at identifying these functions and further enabling engineering efforts that may be widely applicable to other strains.

|
Scooped by
mhryu@live.com
Today, 12:03 AM
|
Streptococcus mutans, the major causative agent of dental plaque and caries, maintains osmotic balance under hyperosmotic conditions by transporting glycine betaine into the cytoplasm via the BusAB transporter system. This mechanism is coordinated by the c-di-AMP-responsive transcriptional regulator BusR, which represses busAB expression in the absence of osmotic stress. In this study, we systematically characterized the function of BusR in S. mutans UA159. Our experiments showed that deletion of busR resulted not only in high expression of busAB but also in upregulated GlcNAc metabolic genes, specifically nagA/nagB and glmS, which are known to be regulated by transcriptional regulator NagR. The ΔbusR strain utilized GlcNAc as a nutrient more efficiently and exhibited a faster growth rate than the wild-type strain. Combined with results from further experimentation, this suggests that, BusR assumes a dual regulatory role under high-osmolarity conditions: it relieves repression of busAB to increase the transport of the osmoprotectant betaine into cytoplasm, and cooperates with NagR to regulate amino sugar metabolism by regulating the transcription of nagA/nagB and glmS. Consistent with the molecular ruler mechanism previously described for BusR homologs from Streptococcus agalactiae, we observe a similar structural basis that enables BusR to mediate precise, c-di-AMP–dependent modulation of gene transcription. This coordinated regulation of osmoprotection and amino sugar metabolism by BusR may give S. mutans a significant advantage for dealing with osmotic stress within the oral environment.

|
Scooped by
mhryu@live.com
February 3, 11:46 PM
|
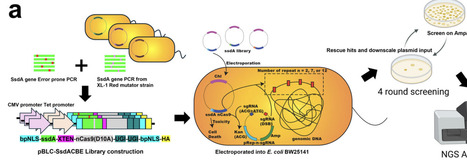
APOBEC1-based cytosine base editors such as BE4max enable base conversion, but many alternative deaminases show low activity and cytotoxicity, especially when miniaturized for delivery. SsdAtox, a DNA deaminase toxin from Pseudomonas syringae that is two-thirds the size of APOBEC1, is attractive for compact base editors but, in native form, shows low C-to-T editing efficiency and high cytotoxicity. Guided by an AlphaFold- and CASTpFold-based alanine scan, we identified K31 as a gatekeeping residue whose substitution enlarges the modeled DNA binding pocket. Site-saturation mutagenesis at K31 produced variants with ten-fold higher activity but increased indel formation. To further enhance activity while reducing indels and cytotoxicity, we developed Trinity-Screen, an E. coli-based three-in-one directed evolution platform that selects for high activity and reduced double-strand break-associated indels. Trinity-Screen revealed four additional DNA-binding positions; combinatorial mutagenesis at these sites generated four- and five-site SsdAtox variants that retained high activity yet showed lower indel rates and rescued bacterial viability. To standardize comparisons, we defined the Base Editor Performance Index (BEPI), which integrates C-to-T conversion and indel frequency. Optimized SsdAtox variants achieved up to 31-fold improvement relative to wild type, outperforming BE4max at multiple endogenous targets and displaying ten-fold lower cytotoxicity in E. coli.
|
 Your new post is loading...
Your new post is loading...




























Identification of gut microbes that efficiently deplete amino acids (aa) -> Identification of gut microbial metabolic genes that deplete aa, Microbiota genes that deplete aa affect host aa homeostasis, Microbiota genes that deplete aa affect host glucose tolerance via peripheral serotonin