 Your new post is loading...

|
Scooped by
?
December 19, 4:35 PM
|
Fungal plant biomass conversion (FPBC) is of great importance to the global carbon cycle and has been increasingly applied for the production of biofuel and biochemicals from lignocellulose. However, the comprehensive understanding of relevant molecular mechanisms in different fungi remains challenging. Here, we comparatively analyzed the transcriptome, proteome and metabolome profile of four ascomycetes and one basidiomycete fungi during their growth on two common agricultural feedstocks (soybean hulls and corn stover). We revealed strong time-, substrate- and species-specific responses at multi-omics levels for the tested fungi, highlighting species-specific carbon utilization approaches and evolutionary adaptation to environmental niches. Notably, a remarkable expressional diversity of lignocellulose degrading enzymes, sugar transporter and metabolic genes, as well as industrially relevant metabolites were identified across different fungi and cultivation conditions. The findings improves our understanding of complex molecular networks underlying FPBC and fungal ecological roles, offering novel insights that can guide future genetic engineering of fungi for valorization of agriculture waste into value-added bioproducts.

|
Scooped by
?
December 19, 4:32 PM
|
Violacein is a natural purple secondary metabolite with a wide range of biological activities including antibacterial, anticancer, antioxidant, and antiparasitic properties, rendering it a highly promising candidate for applications in medicine, agriculture, and food industries. Despite its availability from natural sources, a profound understanding of its production mechanisms has long been lacking. High-level production of violacein has been achieved through integrated strategies, including heterologous expression of its biosynthetic pathway in recombinant strains, enhancement of tryptophan precursor supply, and optimization of fermentation conditions. These approaches offer a flexible and scalable platform for violacein biosynthesis. Furthermore, recent efforts have focused on utilizing agro-industrial waste as a cost-effective and sustainable feedstock to further improve production efficiency and environmental compatibility. This review provides a comprehensive overview of the latest advancements in violacein production, examines the challenges associated with its application, and proposes strategies for optimizing gene expression, refining fermentation protocols, and utilizing low-cost raw materials to facilitate the efficient and sustainable violacein production.

|
Scooped by
?
December 19, 4:27 PM
|
Lignocellulose-derived fuels and chemicals are vital to breaking the world's dependence on fossil fuels. Though plant biomass is notoriously resistant to deconstruction, lignocellulolytic thermophiles are especially adept at degrading its constituent polysaccharides into mono- and oligo-saccharides for catabolism. And many thermophiles, whether lignocellulolytic or not, can be engineered to ferment lignocellulose-derived sugars into valuable fuels and chemicals as part of consolidated bioprocesses. Although the past 20 years have seen major advances in the genetic and metabolic engineering of individual thermophiles, the strategy of co-culturing thermophilic strains as part of synthetic communities has not been well established. Synthetic communities unlock synergistic interactions that outperform monocultures, thereby enhancing product titers, rates, and yields. While limited genetic tools once hindered the development of synthetic thermophilic communities, recent advances now offer robust systems for engineering these industrially relevant organisms. Here, we propose that this expanded genetic malleability enables engineering of 1) transport specialization to reduce substrate competition between strains and 2) division of labor strategies whereby one strain focuses on lignocellulose deconstruction while another strain dedicates metabolic burden for product synthesis. We draw on examples of engineered thermophiles like Clostridium thermocellum, Thermoanaerobacter saccharolyticum, and Anaerocellum bescii to illustrate how these mechanisms have been applied in thermophilic co-cultures. In brief, this perspective outlines design principles to construct effective thermophilic communities for lignocellulose bioprocessing.

|
Scooped by
?
December 19, 4:20 PM
|
Throughout thousands of years, the yeast Saccharomyces cerevisiae has been acting as a cell factory in food production. Lately, it has further functioned as a platform cell factory for the production of a multitude of different compounds, spanning from high-volume fuels to high-value pharmaceuticals. The past decade has witnessed the fact that the innovative tools in synthetic biology have driven the rapid progress of the yeast cell factory, enabling us to edit the genetic systems of organisms efficiently and "reprogram" elements or systems such as genes, circuits, pathways, and networks of organisms. Here, we will offer a brief review that highlights the most recent significant advances and perspectives regarding the innovative tools in yeast synthetic biology. These tools encompass genome editing tools, computational tools, adaptive laboratory evolution, and the standardization of biological DNA parts, with the intention of providing a practical guide for the implementation of novel, effective, and efficient development of the customized yeast cell factory.

|
Scooped by
?
December 19, 4:13 PM
|
Class 1 CRISPR–Cas systems utilize multi-subunit effector ribonucleoprotein complexes to identify and target DNA. Upon recognition, type I systems recruit the helicase/nuclease Cas3 for DNA degradation, while type IV-A systems use the helicase CasDinG for transcriptional repression. Here, we developed two recombinant class 1 CRISPR–Cas genome editing tools for inducing large genomic deletions: the compact type I-Fv (also termed I-F2) system from Shewanella putrefaciens and the type IV-A1 system from Pseudomonas oleovorans. In the latter, CasDinG was engineered to include a C-terminal HNH nuclease domain, conferring DNA cleavage activity and enabling analysis of CasDinG processivity. Whole-genome sequencing of Escherichia coli BL21-AI was used to monitor genome reduction and DNA repair mechanisms in response to CRISPR–Cas-induced damage. Small deletions were flanked by microhomologies, consistent with repair via alternative end joining, whereas deletions larger than 10 kb consistently terminated at nearby IS1 elements, implicating these sequences in the repair process. This study introduces compact type I and engineered type IV-A genome editing tools with distinct protospacer-adjacent motif requirements and provides new insights into CasDinG evolution and the DNA repair pathways engaged during CRISPR–Cas-mediated genome editing.

|
Scooped by
?
December 19, 2:00 AM
|
Rhizosphere microorganisms are known to be able to modify the plant's ability to resist abiotic stresses. It is, however, difficult to modify microbial communities to improve plant phenotypes. Here we tested if a rhizosphere microbial community from a water-stress naive soil could be modified by adding DNA extracted from soils with a water stress history. Six-week-old wheat plants growing under low or high-water availabilities were inoculated with DNA extracted from soils with contrasting long-term histories of water availability - one continuously and the other intermittently exposed to water deficit. The fate of the inoculated DNA in the rhizosphere microbial communities was assessed by shotgun metagenomics. Putatively transferred inoculum genes were disproportionately found in the Acidobacteria and Bacteroidetes and belonged to functional category such as antibiotics, biofilm, and carboxylates metabolism, among others. These functional categories were shared by pre-inoculation laterally transferred genes in the recipient soil, highlighting their usefulness for life in soil. The "continuous" inoculum reduced the stress levels of wheat under reduced soil water content, suggesting that the natural genetic transformation of the rhizosphere community can feedback to the plant. Altogether, we are providing evidence for an ecological mechanism that could be harnessed to modify plant-associated microbial communities and help plants sustain water stress.

|
Scooped by
?
December 19, 1:56 AM
|
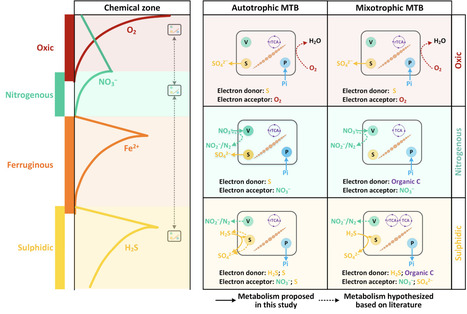
Magnetotactic bacteria (MTB) are capable of accumulating and storing intercellular pools containing phosphorus, sulfur, nitrogen and carbon. Yet, the metabolic pathways connected with their intracellular storage of polyphosphate, elemental sulfur, nitrate, and calcium carbonate, and the environmental influence on MTB inclusions and their quantitative contribution to sedimentary chemical budgets remain underexplored. Using a combination of chemical characterization, ultrastructural and compositional analyses, and phylogenetic and genomic insights into microbial assemblages, we investigated the influence of geochemical parameters on the diversity, ecophysiology, and distribution of MTB in freshwater and brackish sediments. Special focus is given to intracellular inclusions and their metabolic pathways to uncover functional traits and ecological roles in elemental cycling. Of the 118 examined MTB cells, polyphosphate granules (75%) and nitrate-containing vacuoles (67%) were the most common inclusions followed by sulfur globules (25%) and calcium carbonate granules (8%). The intracellular phosphorus, nitrogen, and sulfur stored in MTB cells were conservatively estimated to account for 0.60%, 0.91%, and 0.23% of the total sedimentary P, N, and S in the investigated freshwater sediments, respectively. The ubiquitous nature and important ecological role of MTB can be explained by their ability to sequester chemical elements into intracellular inclusions, giving them a metabolic advantage in dynamic chemically stratified environments. The extraordinary phylogenetic diversity of MTB, coupled with their capability to hyperaccumulate and store a wide range of elements and compounds, represents a significant resource for biotechnology innovation.

|
Scooped by
?
December 19, 1:45 AM
|
The Gene Ontology (GO) knowledgebase https://geneontology.org is a comprehensive resource describing the functions of genes. The GO knowledgebase is regularly updated and improved. We describe here the major updates that have been made in the past 3 years. The ontology and annotations have been expanded and revised, particularly in several areas of biology: cellular metabolism, multi-organism interactions (e.g. host-pathogen), extracellular matrix proteins, chromatin remodeling (e.g. the “histone code”), and noncoding RNA functions. We have released version 2 of a comprehensive set of integrated, reviewed annotations for human genes, which we call the “functionome.” We have also dramatically increased the number of GO-CAM models, with over 1500 models of metabolic and signaling pathways, primarily in human, mouse, budding and fission yeast, and fruit fly. Finally, we discuss our current recommendations and future prospects of AI in the use and development of GO.

|
Scooped by
?
December 19, 1:41 AM
|
Colouration of textiles is responsible for 3% of global greenhouse gas emissions and 20% of global wastewater generation. An estimated 20% of the dyes and pigments used in textile colouration is lost to wastewater. Synthetic dyes and pigments, along with many of the chemicals added to their formulations, are nonbiodegradable, persistent, and bioaccumulative in aquatic ecosystems, posing signi cant risks throughout the food chain. Regulatory bodies in both Europe and the United States are continuing to ban, restrict, and phase out many synthetic dyes and pigments. The EU recently banned a significant number of common textile dyes known to release carcinogenic compounds, while in the United States, 8 common synthetic food dyes are being targeted for immediate ban in the US food supply, with the FDA planning to phase out all remaining synthetic food dyes. While bio-based alternatives are available on the market, they are currently more expensive, restricting their application to niche product applications that can absorb the price premium. The total market size for dyes and pigments is more than US$ 50 billion and is growing at a rate of 3–10% annually. The ‘cost of being unsustainable’ is increasing rapidly, particularly in the EU, as new regulations make the use of hazardous chemicals more difficult and expensive. This is driving a shift toward the use of more sustainable chemicals.

|
Scooped by
?
December 19, 1:31 AM
|
Recent efforts in bottom-up synthetic biology focus on fabricating programmable biological units that can be viewed as synthetic cells. Combining microfluidic techniques with cell-free protein expression systems defines the geometrical limits of the synthetic cell (e.g. microfluidic compartments, droplets, vesicles) and facilitates communication pathways to distribute functions over an assembly of synthetic cells. In this review, we describe and compare the different strategies implemented to reconstitute cell–cell communication among synthetic cells. We focus especially on various experimental setups of microcompartmentalization containing a cell-free expression system and genetic material. We highlight efforts to develop and engineer different modes of communication among the synthetic cells in different forms, varying by the degree of permeability, resource renewal, stability, and scalability, and how these influence the trade-off between programmability and biomimicry. We then summarize recent progress in the realization of different stages of communication (signaling, processing, and output generation) by genetic circuits, holding great promise for applications in synthetic biology and biotechnology.

|
Scooped by
?
December 19, 1:27 AM
|
Single-stranded RNA (ssRNA) can activate the mechanosensitive ion channel Piezo1 in the gut. However, its source and role in colorectal cancer (CRC) remain unclear. In the present study, we demonstrate that ssRNA within fecal extracellular vesicles (FEVs) derived from bacteria, particularly those susceptible to lysis by ursodeoxycholic acid (UDCA), can suppress CRC progression via Piezo1 activation. Gut-specific Piezo1-knockout mice developed more tumors following CRC induction. Similarly, Piezo1-deficient CRC cell lines exhibited increased proliferation with upregulated Wnt/β-catenin signaling. Mechanistically, Piezo1 activation downregulated the transcription factor Zfp281, decreasing the expression of its target gene Lgr5 and dampening Wnt/β-catenin signaling. Notably, oral UDCA administration enhanced FEV rupture, increasing luminal “naked” ssRNA and mitigating high-fat diet-induced CRC in vivo. These findings identify the bacterial ssRNA-Piezo1 axis as a potential therapeutic target in CRC.

|
Scooped by
?
December 19, 1:19 AM
|
Under severe nutrient-limiting conditions, Bacillus subtilis is able to form highly resilient endospores for survival. However, to avoid this irreversible process, it employs an adaptive strategy termed cannibalism, a form of programmed cell death, to outcompete siblings and delay sporulation. One of the three cannibalism toxins, the epipeptide EPE, is encoded by the epeXEPAB operon. The pre-pro-peptide EpeX undergoes post-translational modification and processing to be secreted as the mature EPE toxin. While EPE production is tightly regulated at multiple levels, this study focuses on the post-transcriptional control by the small regulatory RNA FsrA, which is transcriptionally regulated by the global iron response regulator Fur. Electrophoretic mobility shift assays and RNA structure probing revealed two binding sites of FsrA within the intergenic region between epeX and epeE flanking the annotated epeX terminator structure and potentially interfering with RNA stability and epeXEP expression. Reporter assays revealed decreased levels of EPE-dependent stress response in the absence of FsrA, indicative of a positive FsrA effect on gene expression under iron-limited conditions; in contrast to the normally inhibitory activity of FsrA. Together, our findings suggest that under iron starvation, FsrA promotes RNA processing and enables epeE translation, ultimately enhancing EPE production.

|
Scooped by
?
December 19, 12:30 AM
|
Base editors enable precise genome modification but are constrained by bystander edits that limit their applicability. Existing strategies to enhance precision often compromise efficiency and remain highly sequence dependent. Here we present a parallel engineering approach that optimizes both guide RNAs and the deaminase enzyme to minimize bystander editing without sacrificing activity. We designed a library of 3′-extended guide RNAs and identified context-dependent variants that improved specificity. Using a precision-driven phage-assisted evolution system and protein language models, we evolved adenine base editor variants two- to threefold more precise than adenine base editor ABE8e while maintaining high efficiency across a library of thousands of human pathogenic contexts in vitro. Our findings establish a scalable framework for precision engineering of base editors, addressing a major challenge in genome editing. Base editors are made more precise through deaminase and gRNA engineering.
|

|
Scooped by
?
December 19, 4:33 PM
|
A great number of multifactorial diseases, including neoplastic, metabolic, and autoimmune diseases, have been associated with microbiota dysbiosis. Recently, there has been an increasing understanding of the importance of microbiome and their impact on human health. Advances in synthetic biology have led to the development of probiotics as diagnostic tools and disease treatment approaches. In this review, we briefly summarize recent examples of engineered probiotic-based therapeutics in human diseases, including cancers, gastrointestinal disorders, infectious diseases, and metabolic disorders. Finally, we discuss the challenges and opportunities in developing engineered probiotics for disease treatments.

|
Scooped by
?
December 19, 4:30 PM
|
Plant-microbe interactions are critical to ecosystem resilience and substantially influence crop production. From the perspective of plant science, two important focus areas concerning plant-microbe interactions include: 1) understanding plant molecular mechanisms involved in plant-microbe interfaces and 2) engineering plants for increasing plant disease resistance or enhancing beneficial interactions with microbes to increase their resilience to biotic and abiotic stress conditions. Molecular biology and genetics approaches have been used to investigate the molecular mechanisms underlying plant responses to various beneficial and pathogenic microbes. While these approaches are valuable for elucidating the functions of individual genes and pathways, they fall short of unraveling the complex cross-talk across pathways or systems that plants employ to respond and adapt to environmental stresses. Also, genetic engineering of plants to increase disease resistance or enhance symbiosis with microbes has mainly been attempted or conducted through targeted manipulation of single genes/pathways of plants. Recent advancements in synthetic biology tool development are paving the way for multi-gene characterization and engineering in plants in relation to plant-microbe interactions. Here, we briefly summarize the current understanding of plant molecular pathways involved in plant interactions with beneficial and pathogenic microorganisms. Then, we highlight the progress in applying plant synthetic biology to elucidate the molecular basis of plant responses to microbes, enhance plant disease resistance, engineer synthetic symbiosis, and conduct in situ microbiome engineering. Lastly, we discuss the challenges, opportunities, and future directions for advancing plant-microbe interactions research using the capabilities of plant synthetic biology.

|
Scooped by
?
December 19, 4:23 PM
|
Approaches are needed to reduce the impact of climate change and provide freshwater. Synthetic biology, and plant synthetic biology specifically, is in an exceptional position to address these needs. Plants are powerful synthetic biology platforms as they derive their energy from the sun (photosynthesis) while capturing the potent greenhouse gas, carbon dioxide. Historically, plants were used in specialized circumstances to provide and store freshwater. Even today many mangrove species can filter seawater while some plants can store massive amounts of water. I propose that starting from nature's own design principles we could engineer plants to filter seawater and/or store freshwater. Moreover, synthetic biologists could derive components found in diverse life forms, modified by water's evolutionarily adaptive properties, to provide source material for engineering plants to filter and transport water. By combining components and inspiration from nature, with advanced synthetic biology tools such as computational protein design and directed evolution, we have the knowledge and technology to make an impact on climate change. Synthetic biologists could engineer plant platforms with the ability to filter saltwater while producing freshwater thus providing a powerful and sustainable means to address freshwater needs while reducing the impact of climate change. With creativity and time, plant synthetic biology could thus help provide sustainable solutions.

|
Scooped by
?
December 19, 4:17 PM
|
White biotechnology stands as a major sustainable alternative to address pressing environmental issues arising from our heavy dependence on petrochemical synthesis. However, reaching this goal, both technologically and economically, will take time, resources and money. A major reason is within the biological system itself, as it has evolved into a bow-tie structure in which carbon and energy are converted, via highly regulated, complex and interconnected metabolic networks, into cellular components for growth and homeostasis. This objective is fundamentally at odds with that of biotechnology, which aims to convert carbon and energy into bioproducts. Engineering of microorganism using systems and synthetic biological systems tools has been developed to provide a compromise between these two objectives. However, these genetic and metabolic interventions have revealed often unexpected physiological behaviors, in part due to the fact that a large proportion of metabolic enzymes are catalyzing other reactions than those for which they were evolved. While this promiscuity is the source of an underground metabolism that can prove very advantageous in building high-performance production routes, it is also responsible for loss of yield and production due to metabolic disturbances, negative cross-talks between natural and heterologous pathways as well as it is at the onset of metabolic damages. Identifying these promiscuous enzymes and thus anticipating their opportunities or weaknesses in engineering microbial cell factories for bioproduction is a major challenge in order to improve their performance. It is foreseen that machine learning tools operating on databases continuously fed by genetic, metabolic, enzymatic and fermentation processes data can help to overcome these challenges and provide a better understanding of the physiological functioning of the microbial system.

|
Scooped by
?
December 19, 4:07 PM
|
Owing to the complexity of living cells, multicellular systems exhibit heterogeneity at both the macro (different cell types) and the micro (molecular states within a cell type) levels. Traditionally, such heterogeneity has challenged the yield and quality of stem cell-derived cell therapy manufacturing. Here, we argue that heterogeneity can instead be harnessed as a design feature in the quality-by-design toolkit to optimize cell therapy yield and quality, thereby improving bioprocess robustness. We propose a framework for mapping input cell state to output cell fate using systems and synthetic biology tools. This framework can be used to define material and critical quality attributes at the molecular level that better predict drug safety and efficacy. By understanding the sources and consequences of heterogeneity, we can harness it to conquer complex cell therapy manufacturing and bring it to the level of robustness currently only achieved for biologics and small molecules.

|
Scooped by
?
December 19, 1:58 AM
|
Type IV secretion systems (T4SS) enable the spread of antibiotic resistance and other virulence factors. In Gram-positive bacteria, T4SSs have long been thought to lack VirB2-like proteins that form conjugative pili and instead rely on adhesins for cell-cell contacts. Yet, it has remained unclear how subsequent DNA transfer (conjugation) is physically mediated. Here we identify a VirB2-like protein, PrgFB2, from the clinically isolated conjugative plasmid pCF10 in Enteroccocus faecalis and show that it is essential for conjugation. Structural modeling confidently predicts a pilus-like assembly for PrgFB2. We validate this prediction through mutagenesis, conjugation assays, and targeted chemical labeling. By combining various machine learning bioinformatic techniques, we analysed >1000 Gram-positive conjugative plasmids from diverse species, including major pathogens, and identified pili forming VirB2-like proteins in almost all of them. Our findings overturn the prevailing view that Gram-positive T4SSs lack pili. This discovery provides a new framework for understanding horizontal gene transfer and highlights critical targets for combating antimicrobial resistance and virulence in Gram-positive bacteria.

|
Scooped by
?
December 19, 1:51 AM
|
Prime editing (PE) enables the precise installation of intended base substitutions, small deletions or small insertions into the genome of living cells. While the use of Cas9 nickase can avoid DNA double-strand breaks (DSB), undesired insertions and deletions (indels) often accompany the correct edits, particularly when PE activity increased. Here we show that the anti-CRISPR (Acr) protein AcrIIA5 can significantly enhance PE activity by up to 8.2-fold while markedly reducing byproduct indels. Further investigation reveals that AcrIIA5 can promote PE across various approaches (PE2, PE3, PE4, PE5, and PE6), edit types (substitutions, insertions and deletions), and endogenous loci. Mechanistically, AcrIIA5 appears to inhibit the re-nicking activity of PE complex rather than enhancing the core editing machinery itself, suggesting a distinct mode of interaction with Cas9. Overall, we demonstrate that a known “inhibitor” Acr protein can unexpectedly acting as an “enhancer” of CRISPR/Cas-based genome editing, providing an effective strategy to optimize PE specificity and activity. Prime editing enables precise genetic modification but often suffers from unwanted byproducts. Here, authors show that the anti-CRISPR protein AcrIIA5 unexpectedly enhances prime editing efficiency while reducing unintended indels, offering an effective strategy to improve activity and specificity.

|
Scooped by
?
December 19, 1:43 AM
|
Directed evolution facilitates functional adaptations through stepwise changes in sequence that alter protein structure. While most campaigns yield solutions that maintain the framework of a rigid protein architecture, a few have produced enzymes with more notable structural differences. One example is a polymerase that was evolved to synthesize threose nucleic acid (TNA) with near-natural activity. Understanding how this enzyme arose provides a model for studying pathways that guide enzymes toward more productive regions of the fitness landscape. Here, we trace the evolutionary trajectory of an unnatural polymerase by solving crystal structures of key intermediates along the pathway and evaluating their biochemical activity. Contrary to the view that fidelity is a product of increased catalytic efficiency, we find that accuracy and catalysis are decoupled activities guided by separate ground-state and transition-state discrimination events. Together, these results offer a glimpse into the forces responsible for shaping the emergence of new enzyme functions. Engineering polymerases to synthesize alternative genetic polymers remains a challenging problem in synthetic biology. The current study offers insights into the structural and biochemical changes responsible for improving the fidelity and catalytic activity of a laboratory evolved TNA polymerase.

|
Scooped by
?
December 19, 1:35 AM
|
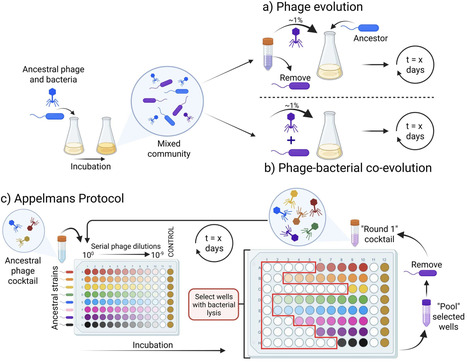
Bacteriophages are viruses that selectively prey on bacteria. Their use in treating antimicrobial-resistant bacterial infections is steadily increasing due to the need for alternative therapies. The application of phage therapy is not without its challenges, including difficulties associated with isolating phages against a target strain, the limited infectivity of a phage, the cost and complexity of producing well-characterized phage stocks, and the emergence of phage resistance. The directed adaptation of phage to a specific bacterial target, also known as ‘phage training’, leverages the natural evolutionary capacity of phages and can be used to bolster their bacterial killing abilities. Phage training dates back almost as far as phage therapy itself, being used to expand the therapeutic use of phages. Numerous reports showcase the success and benefits of phage training in vitro and its potential to operate effectively within the framework of phage therapy. However, the time needed to train a given phage, followed by genotypic and phenotypic characterisation of both pre- and post-trained phages, is a major limitation. Here, we explore oversights of the phage training process and propose some considerations and solutions to help drive the field forward to enable its feasible integration into phage therapy.

|
Scooped by
?
December 19, 1:30 AM
|
Persister cells survive any severe stress including antibiotics, starvation, heat, oxidative conditions, and phage attack, by entering a dormant physiological state. They arise without genetic change and can resume growth once the stress is removed and nutrients are available. Critically, upon resuscitation, persister cells can reconstitute infections. Although it is known that persister cells resuscitate in proportion to their ribosome content, it has remained unclear whether ribosome levels also influence the formation of persister cells. Here, we used fluorescence-activated cell sorting (FACS) to fractionate exponentially growing cells into four populations spanning low to high ribosome levels and demonstrated that cells with low ribosome content form persister cells approximately 80-fold more frequently than cells with population-average ribosome levels. These findings show that persister cell formation is inversely proportional to ribosome abundance. Cells with low ribosome levels are less metabolically-active and therefore less capable of initiating a stress response like most cells; instead, they become dormant.

|
Scooped by
?
December 19, 1:21 AM
|
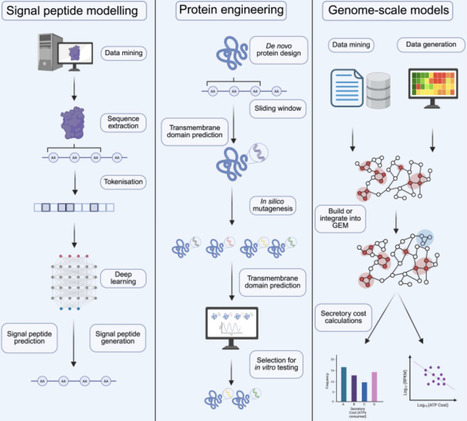
Protein secretion in mammalian cells is the active transport of proteins from the cytoplasm to the extracellular space. It plays a fundamental role in mammalian physiology and signaling, as well as biotherapeutics production and cell and gene therapies. The efficacy of protein secretion, however, is impacted by features of the secreted protein itself, and the host-cell machinery that supports each step of the secretion process. High-throughput techniques such as microfluidics, cell display, and cell encapsulation assays for the study and engineering of secreted proteins are transforming biomedical knowledge and our ability to modulate protein secretion. In addition, computational advances, including signal peptide modeling, whole-protein machine learning models, and genome-scale simulations, are opening new pathways for rational design of protein secretion. Here, we highlight recent developments in secretion engineering that are leading to the convergence of high-throughput experimentation and machine learning methods and can help address current challenges in bioproduction and support future efforts in cell and gene therapy while enabling new modalities.

|
Scooped by
?
December 19, 1:15 AM
|
Extracting fungal hyphae with their naturally associated microbiota from soil samples presents a significant challenge due to their small size, typically in the micrometer range, and the formation of dynamic fungal networks. We combined elements of previous protocols and automated the wet-sieving steps of the methodology to efficiently extract fungal hyphae from various soil types, including natural loamy soils. This approach reduces manual handling, minimizes operator-dependent variability, and shortens processing time by up to 2.5-fold. Unlike earlier methods that require sand or glass bead supplementation, which can introduce artificial conditions and limit large-scale field applications, our Sieving and Sucrose Centrifugation (SSC) method avoids these drawbacks. The SSC technique enables both quantification of hyphal length density (HLD) and, importantly, preserves surface-associated microbes for downstream analyses. Among the tested methods, SSC yielded the highest hyphal length density. Using a combination of microscopy, molecular techniques, and next-generation sequencing (NGS), we demonstrate that this method allows targeted study of bacteria tightly attached to fungal hyphae. Furthermore, the SSC approach effectively enriched fungal hyphae from a highly diverse soil community, establishing a dependable tool for advancing research on fungal hyphae as microbial hotspots in soil ecosystems.
|
 Your new post is loading...
Your new post is loading...





























aspergillus