 Your new post is loading...

|
Scooped by
mhryu@live.com
Today, 5:09 PM
|
Agrobacterium-mediated transformation relies on binary vectors in which T-DNA and virulence genes are maintained on separate replicons. While Golden Gate cloning has become standard for T-DNA assembly, no modular framework exists for systematic construction of Agrobacterium vector backbones. Here, we present BackBone Builder (B3), a Golden Gate-based platform for combinatorial backbone assembly. B3 uses the Type IIS enzyme PaqCI to minimize domestication and enables one-pot assembly of nine backbone modules plus a selectable cloning cassette. The system is compatible with GreenGate and remains independent of downstream cloning strategies. We generated a library of 42 backbone components, supporting a theoretical design space exceeding 370,000 constructs. A 4 x 4 origin-of-replication (ORI) matrix combining four Escherichia coli and four Agrobacterium ORIs assembled with 100% efficiency and functioned robustly in bacterial and plant contexts. Reporter expression reflected expected ORI-dependent patterns in E. coli, Agrobacterium, and Nicotiana benthamiana. A B3-derived maize transformation backbone achieved stable transformation efficiencies comparable to established vectors. B3 establishes a standardized and extensible framework for rational engineering of Agrobacterium binary vector architecture.

|
Scooped by
mhryu@live.com
Today, 5:05 PM
|
Enzymatic recycling of polyethylene terephthalate (PET) provides a sustainable alternative to conventional PET waste treatment, but the scalable production of PET-degrading enzymes remains a challenge. A key bottleneck is intense cell lysis during production, caused by the PET hydrolases attacking the host cell membrane. This lysis leads to elevated extracellular DNA content, increased viscosity, excessive foaming, reduced oxygen transfer during fermentation, and difficulties in downstream processing such as filtration and centrifugation. In this study, we explored two strategies to address these issues: (1) the addition of nucleases during fermentation, and (2) co-cultivation of PETase- and nuclease-expressing E. coli strains in varying inoculum ratios. To stabilize population distribution during the production phase of co-cultivation, we employed a growth-decoupled expression system. Both strategies reduced extracellular DNA, lowered broth viscosity, and improved cell settling and foam control, while maintaining or even enhancing PET hydrolase activity and titer. While adding nuclease to produce PETase may increase production costs, co-cultivation provides a promising alternative to simplify processing. Our findings offer a scalable and cost-efficient strategy for producing lysis-prone enzymes, enabling robust fermentation processes for industrial enzymatic PET recycling.

|
Scooped by
mhryu@live.com
Today, 4:57 PM
|
Isoprenoids play vital roles in all domains of life, from beta-carotene in bacteria to heme in humans. Two distinct metabolic pathways have evolved to synthesize the critical precursor of all mature isoprenoids: the mevalonate (MEV) and the methylerythritol phosphate (MEP) pathways. Here, we quantify the extensive inter- and intra-genus heterogeneity in the usage of these two pathways with particular emphasis on rare bacteria that encode both, or neither, pathways. Furthermore, MEP intermediates themselves have non-isoprenogenic roles that may underlie evolutionary pressures driving pathway diversification. Understanding isoprenoid biosynthesis in bacteria offers new avenues toward more sustainable engineering of economically relevant molecules in microbes.

|
Scooped by
mhryu@live.com
Today, 4:29 PM
|
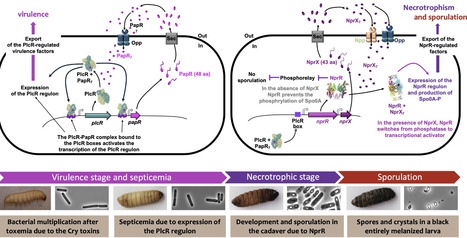
Within the vast Bacillus cereus group, two bacterial species have stood out for over a century: Bacillus anthracis for its pathogenicity to mammals, and Bacillus thuringiensis for its remarkable and economically exploitable activity against invertebrates. One hundred years of extensive research around the world have unraveled the sophisticated mechanisms that make B. thuringiensis a formidable weapon designed to kill insects, exploiting them as an ecological niche for its proliferation. Evolution has led to the selection of a great diversity of highly specific toxins targeting a wide range of insects and nematodes. Bacteria have developed transcriptional, post-transcriptional, and post-translational mechanisms that enable the massive production of these toxins as crystalline inclusions. Virulence and adaptation factors, together with regulation systems, have also been selected to enable the bacterium to make the most of the ecological niche provided by insects. In addition to their interest in the bacterium, the biological tools and processes developed by B. thuringiensis can be exploited by mankind to create insect-resistant plants, overproduce proteins, crystallize them, and gain a better understanding of the microbial world. All the research carried out on B. thuringiensis over the last century has made this bacterium a remarkable study model and biotechnological resource, revealing all the subtlety and power of the mechanisms that a microorganism has been able to acquire in the course of its evolution.

|
Scooped by
mhryu@live.com
Today, 4:05 PM
|
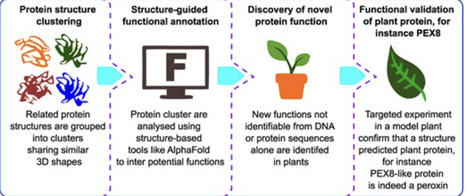
In a recent Breakthrough Report, Jiarong Chen, Yanlei Feng, Yuchan Zhang, and colleagues (Chen et al. 2026) used a structure-based approach that goes beyond the traditional sequence alignment to systematically annotate previously uncharacterized plant proteins and predict their functions (Fig. 1). Analyzing protein structures from 17 flowering plant species, the team clustered >550 K predicted proteins into >170 K groups based on structural similarity. From these, they identified more than 3,000 clusters where structural alignment revealed conserved architectures that sequence-based methods missed. Further refinements narrowed this set to 1,292 high-quality protein clusters, of which 246 clusters were broadly conserved across many plant species. By manual curation, the team ultimately highlighted 120 protein clusters widely present across plants and with predicted functions that could not be inferred from sequence data alone.

|
Scooped by
mhryu@live.com
Today, 3:38 PM
|
This study provides the first clear evidence that edible mushrooms, such as Lentinula edodes (shiitake), Pleurotus ostreatus and Pleurotus eryngii, can generate carbon monoxide (CO) as part of their metabolic activity—independent of bacteria, illumination or oxygen limitation. Systematic measurements of CO and CO2 emissions were performed over 60 days using multiple fungal species, substrates and growth conditions. Microscopy observations (light, scanning and transmission electron microscopy) confirmed no extracellular and intracellular bacterial endosymbionts involved, supporting a fungal genesis of CO. CO emission patterns showed a parabola-shaped curve, correlating with CO2 levels regardless measurements by gas-analyser or GC–MS and peaking during full mycelial colonization. Shiitake mushrooms grown on birch substrate released the highest CO compared to alder and aspen substrates and P. ostreatus and P. eryngii. These findings suggest that fungal respiration contributes to CO dynamics more than previously recognized and highlight the need for further research into its mechanisms and environmental and occupational health implications.

|
Scooped by
mhryu@live.com
Today, 11:14 AM
|
Cas9-based genome engineering is a powerful tool for yeast strain development, but its use in the food industry is limited due to GMO concerns. As a non-GMO alternative, adaptive laboratory evolution (ALE) was applied to enhance trehalose accumulation, a stress protectant, in Saccharomyces cerevisiae. To avoid ethanol-centric metabolism and promote storage carbohydrate production, ALE was conducted under nitrogen limitation with ethanol as the sole carbon source. The evolved strain 65EV showed a 2.3-fold increase in trehalose (11.51% vs 5.25%), resulting in enhanced cell viability (77.7% vs 5.11%) and bread loaf volume (107.2 mL vs 61.5 mL) after freeze/thaw stress. Amino acid profiling revealed distinct metabolic shifts, including elevated intracellular proline and extracellular glutamate. Whole-genome sequencing and reverse engineering identified unique mutations, TSL1 (V887A) and SSA2 (F105L), associated with trehalose regulation. These findings demonstrate potential of ALE as a non-GMO strategy for improving yeast performance in food applications.

|
Scooped by
mhryu@live.com
Today, 11:08 AM
|
Maintaining proper redox conditions is essential for protein stability and function. In cell-free protein synthesis, reducing agents, such as dithiothreitol and reduced glutathione, are commonly added to mimic the cytosolic environment and prevent unwanted oxidation. The PURE system, which is a fully reconstituted protein synthesis system, also contains reducing agents. Here, we systematically examined how reducing agents affect the protein synthesis in the PURE system. We found that the reducing activity of dithiothreitol decreased during prolonged reactions, leading to the formation of disulfide bonds in synthesized proteins. Dissolved oxygen and contaminating metal ions were identified as major factors causing this loss of activity. Based on these findings, we developed a method to maintain reducing conditions throughout the reaction, ensuring consistent protein quality. Our results provide new insights into redox regulation in cell-free systems and offer a practical strategy for the efficient synthesis of functional proteins, with potential applications in biotechnology and therapeutic protein production.

|
Scooped by
mhryu@live.com
Today, 11:00 AM
|
Annotation of genes and transcripts is a key prerequisite for understanding the information that is encoded in newly sequenced genomes. One source of information suited for this purpose is RNA-seq data mapped to the respective genome sequence. RNA-seq-based approaches for transcript reconstruction generate transcript models from these data by combining regions of contiguous coverage (exons) and split read mappings (introns). Understanding phenotypes as a consequence of proteins encoded in a genome further requires the annotation of coding sequences within transcript models. We present GeMoSeq, a novel approach for transcript reconstruction from RNA-seq data that combines combinatorial enumeration of candidate transcripts with heuristics for splitting candidate transcripts into regions of contiguous coverage and subsequent likelihood-based quantification. Prediction of coding sequences is an integral part of the GeMoSeq algorithm. We benchmark GeMoSeq against previous approaches using a large collection of public RNA-seq data for seven species. For the majority of species, we observe an improved prediction performance of GeMoSeq, especially on the level of coding sequences and for species with dense genomes. We combine GeMoSeq with the homology-based approach GeMoMa to re-annotate two recently sequenced genomes of Nicotiana benthamiana lab strains, which illustrates the main purpose of GeMoSeq: the initial annotation of newly sequenced genomes with protein-coding genes.

|
Scooped by
mhryu@live.com
Today, 10:31 AM
|
Extracellular electron transfer (EET) is essential for electroactive microbes’ physiology and biotechnological applications. Many such microbes are Gram-negative bacteria, in which EET must cross two membranes and the periplasm, necessitating spatial and temporal collaborations of various EET proteins that reside at different cellular compartments, for which little is known. Using single-molecule/single-cell-level fluorescence microscopy and electrochemical manipulations, we discover that in the electroactive bacterium Shewanella oneidensis, the inner-membrane electron-transfer hub protein CymA undergoes spatial reorganization into localized regions during active EET with dispersed formation dynamics, subsequently driving the colocalization of its direct electron-transfer partners in the periplasm. Correlated single-cell-level photoelectrochemistry-fluorescence microscopy further proves the critical function of CymA reorganization in enabling EET. A multitude of evidence suggests that CymA reorganization stems from biomolecular condensate formation, likely initiated by association with menaquinone-rich inner-membrane domains. These orchestrated spatiotemporal protein dynamics extend the functional roles of biomolecular condensates to include facilitation of EET in bacteria, with broader implications for cellular processes. Extracellular electron transfer (EET) in bacteria requires the spatiotemporal coordination of many proteins. Here, authors show that the inner-membrane protein CymA reorganizes spatially into condensates and drives the colocalization of partner proteins to enable EET in Shewanella.

|
Scooped by
mhryu@live.com
Today, 10:07 AM
|
Genome annotation captures the essence of a genome by cataloguing its genes, transcripts, proteins and other functional elements of the DNA sequence. Accurate annotation serves as the foundation for a wide range of downstream analyses and discoveries, ranging from basic biology to an understanding of the linkage between genes and disease. Over the past two decades, advances in high-throughput sequencing techniques have enabled faster and more accurate capture of diverse genomic features, generating data at an unprecedented scale. Concurrently, computational methods for translating these data into evidence for genome annotation have steadily improved, leading to better automated genome annotation systems. As such, the growing number of sequenced genomes provides a positive feedback loop, in which database searches become more effective and shared sequence patterns emerge more clearly. These advances are promising steps towards annotating the functions of many poorly understood genes, particularly non-coding RNA genes, for which more research is needed. In this Review, Ji et al. overview how rapidly advancing experimental and computational methods are enabling improved and automated annotation of gene structure and function, providing researchers with genome annotation resources of unprecedented scale and resolution.

|
Scooped by
mhryu@live.com
Today, 9:34 AM
|
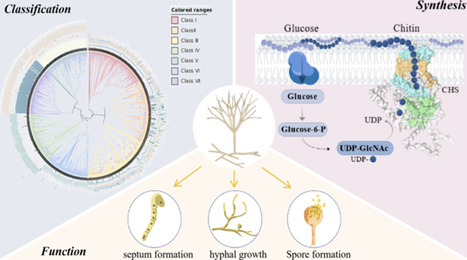
Fungi contain the most complex chitin synthase family, which contributes to chitin biosynthesis and cell wall rigidity. In the past decades, their functions involved in morphological diversity, viability, and virulence of fungi have been well investigated using typical molecular methods such as gene knockout or gene silence, which seems to give a simple aspect of their comprehensive roles. The present review summarized the comprehensive advances of chitin synthases including their classification, their precise structures including catalytic and noncatalytic protein domains, the catalytic mechanisms of chitin biosynthesis and translocation, and roles in cell wall formation, cell separation, and cell viability to provide the references for understanding and guiding the discovery of the novel antifungal drugs targeting the chitin synthases according to their structural basis to offer significant effects and lower resistance development.

|
Scooped by
mhryu@live.com
Today, 1:23 AM
|
Here, we introduce Detectrons, modular biosensors that couples programmable toehold switches with retron-mediated reverse transcription to transduce RNA inputs into unique DNA barcodes. The ability to convert dynamic RNA signals into durable DNA records within living cells unlocks powerful new modes of transcript-based sensing with applications including viral infection detection. Through the construction of a synthetic toehold-retron library and application of machine learning, we uncovered key design principles that improve signal strength and specificity. We applied Detectrons to the multiplexed live-cell detection of specific phage infections, enabling transcript-triggered barcode synthesis and quantitative host susceptibility profiling in pooled bacterial populations. Detectrons are the first RNA-to-DNA transduction system, directly linking transient RNA detection to stable, sequence-encoded DNA outputs. This platform provides a scalable and generalizable strategy for phage screening and for recording transcriptional events in complex bacterial communities.
|

|
Scooped by
mhryu@live.com
Today, 5:08 PM
|
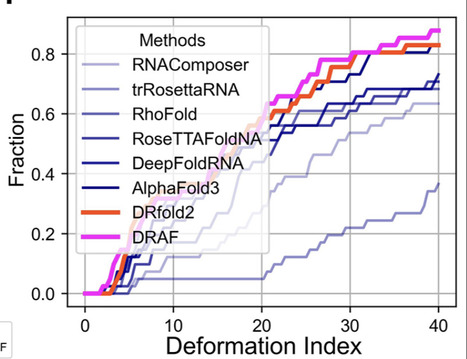
RNA structures are essential for understanding their biological functions and developing RNA-targeted therapeutics. However, accurate RNA structure prediction from sequence remains a crucial challenge. We introduce DRfold2, a deep learning framework that integrates a novel pre-trained RNA Composite Language Model (RCLM) with a denoising structure module for end-to-end RNA structure prediction. Based solely on single sequence, DRfold2 achieves superior performance in both global topology and secondary structure predictions over other state-of-the-art approaches across multiple benchmark tests from diverse species. Detailed analyses reveal that the improvements primarily stem from the RCLM’s ability to capture co-evolutionary pattern and the effective denoising process, with a more than 100% increase in contact prediction precision compared to existing methods. Furthermore, DRfold2 demonstrates high complementarity with AlphaFold3, achieving statistically significant accuracy gains when integrated into our optimization framework. By uniquely combining composite language modeling, denoising-based end-to-end learning, and deep learning-guided post-optimization, DRfold2 establishes a distinct direction for advancing ab initio RNA structure prediction.

|
Scooped by
mhryu@live.com
Today, 5:01 PM
|
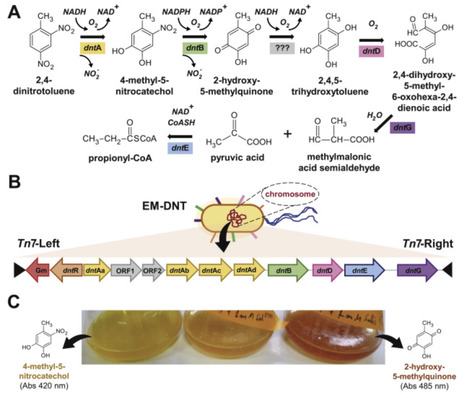
While current genetic tools easily enable transfer of metabolic genes among bacteria, their effective nesting in the recipients depends on biochemical and regulatory compatibilities of the introduced pathway with those of the host. This issue becomes evident in e.g. attempts to engineer bacteria to degrade 2,4-dinitrotoluene (DNT), a xenobiotic compound naturally broken down—if quite ineffectively—by Burkholderia sp. DNT through the so-called dnt route. That despite multiple efforts no strain engineered with a complete set of dnt genes has been able to grow on DNT as sole carbon and nitrogen source suggests that new hosts need to go through a mutual chassis-implant adaptation process for successful degradation of this xenobiotic. To explore a possible roadmap for this to happen we have applied Adaptive Laboratory Evolution (ALE) to a Pseudomonas putida designed to carry an active dnt pathway but initially unable to grow on DNT. Over 315 days of selective subculturing, evolved strains emerged that could metabolize DNT as the sole growth substrate. Genetic and phenotypic analyses of the best-performing isolate revealed a large number of adaptations that improved stress tolerance and fine-tuned to host’s metabolic context to the newly introduced route. These results expose the occurrence of a sort of molecular negotiation between the incoming genes and the pre-existing molecular network of the host before cells entirely integrate the new pathway into their biochemical complement.

|
Scooped by
mhryu@live.com
Today, 4:43 PM
|
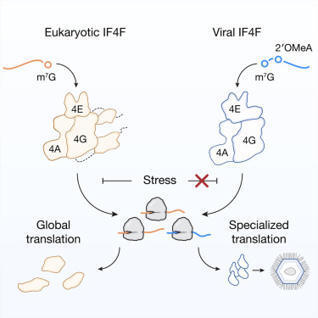
In contrast to living organisms, viruses were long thought to lack protein synthesis machinery and instead depend on host factors to translate viral transcripts. Here, we discover that giant DNA viruses encode a distinct and functional IF4F translation-initiation complex to drive protein synthesis, thereby blurring the line between cellular and acellular biology. During infection, eukaryotic IF4F on host ribosomes is replaced by an essential viral IF4F that regulates viral translation, virion formation, and replication plasticity during altered host states. Structural dissection of viral IF4F reveals that the mRNA cap-binding subunit mediates exclusive interactions with viral mRNAs, constituting a molecular switch from translating host to viral proteins. Thus, our study establishes that viruses express a eukaryotic translation-initiation complex for protein synthesis, illuminating a series of evolutionary innovations in a core process of life.

|
Scooped by
mhryu@live.com
Today, 4:20 PM
|
RNA interference (RNAi) is a crucial biological post-transcriptional gene silencing mechanism where small interfering RNA (siRNA) guides RNA-induced silencing complex (RISC) to bind with messenger RNA (mRNA) thereby silencing it and stopping protein formation. We exploit this process to prevent the formation of harmful proteins by silencing mRNA before it is translated into protein through an effective siRNA. There exists a need to develop a computational model that predicts the effectiveness of siRNA on a given mRNA. Designing a model is challenging, as the data availability is either scarce or biased, and existing models lack generalization ability, even though the parameters to training samples ratio is very high. To overcome these challenges, we introduce RNAiSpline, which incorporates self-supervised pretraining and fine-tuning with Kalmogorov-Arnold Network (KAN), Convolutional Neural Network (CNN), and Transformer Encoder. Evaluation on the independent test dataset yields an ROC-AUC of 0.8175, an F1 score of 0.7717, and Pearson correlation of 0.6032, making RNAiSpline a robust model for siRNA efficacy prediction.

|
Scooped by
mhryu@live.com
Today, 3:57 PM
|
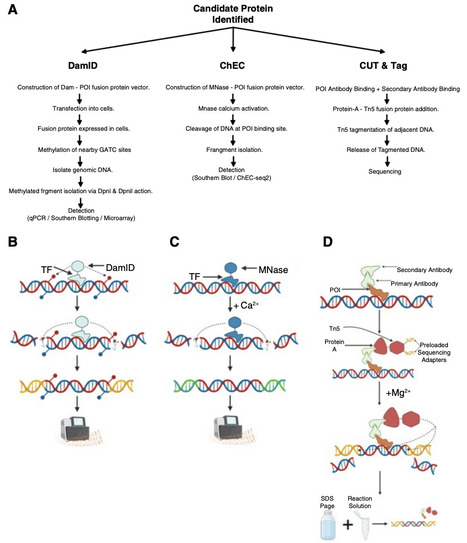
Transcriptional regulation lies at the heart of cellular identity and function, hinging on the precise binding of transcription factors (TFs) and cofactors to gene regulatory elements such as promoters and enhancers. Although it is relatively routine to profile genome-wide DNA binding landscapes of proteins, identifying the specific proteins that bind to, and regulate the transcription of, a particular gene of interest (GOI) remains a persistent experimental and conceptual challenge. This gene-centric question is complicated by the multilayered regulatory environment in which each gene resides, comprising 3D chromatin structure, enhancer–promoter looping, DNA accessibility, histone modifications, and cell state–dependent protein dynamics. In this review, we dissect the strengths, limitations, and biological relevance of current approaches for studying direct protein–DNA interactions, distinguishing between protein-centric and DNA-centric methodologies. We introduce a conceptual matrix of biological relevance, integrating the origin of DNA and protein elements (cis and trans) to evaluate false-positive and false-negative risks across experimental systems. Moreover, we explore how perturbation strategies—gain and loss of function—can complement steady-state profiling to establish causality in gene regulation. By critically examining both established tools and emerging techniques such as genome editing, synthetic chromosomes, and high-resolution imaging, we provide a practical framework for investigators seeking to uncover direct regulators of specific genes. Our goal is to guide the design of experiments that balance biological relevance, sensitivity, and interpretability to ultimately answer a deceptively simple question: What TFs directly regulate the expression of my GOI?

|
Scooped by
mhryu@live.com
Today, 2:53 PM
|
Microbial lipids offer a promising alternative to petrochemicals, but high associated costs and low conversion efficiencies pose barriers to their commercialisation. In particular, sugar-based feedstocks are too expensive for the production of commodity chemicals, and recently attention has turned to volatile fatty acids (VFAs) as a cheaper, more widely available carbon source. Acidogenic fermentation can be used to produce high concentrations of VFAs from municipal and agricultural waste. By harnessing metabolically engineered Acinetobacter baylyi ADP1, the suitability of VFAs as sole carbon sources for wax ester (WE) production was investigated. These studies resulted in the highest WE accumulation in ADP1 achieved to date, at 37% of cell dry weight, and the first reported production of bacterial WEs from a raw, mixed waste stream, utilizing fermentate as the sole carbon source. WE titres of over 160 mg/L from VFAs were achieved, highlighting the unique benefits of mixed feedstocks typically considered problematic for bioproduction. Finally, the potential advantages of employing fermentates rich in longer chain VFAs are explored. In synthetic media, WE titres up to 190 mg/L were achieved, but translation to fermentate was challenging, emphasising the need for continued research in this area.

|
Scooped by
mhryu@live.com
Today, 11:11 AM
|
Lactic acid bacteria (LAB), a group of Gram-positive bacteria widely used in food fermentation, are major producers of bacteriocins─ribosomally synthesized antimicrobial peptides. These peptides have attracted considerable attention not only as natural food preservatives but also for their therapeutic potential in biomedicine. This review outlines LAB-derived bacteriocins, presenting an updated classification (Classes I–III) by structure and genetics, along with their biosynthesis and gene clusters. Furthermore, key production influencers, such as quorum sensing, two-component systems, and culture conditions, are analyzed. Besides, this review also highlights the health benefits of LAB bacteriocins against infections and cancers, achieved via mechanisms like membrane disruption. It also discusses strategies (e.g., metabolic engineering and synthetic biology, combination therapies, and novel purification methods) to enhance efficacy. Despite persistent challenges in yield optimization and clinical translation, this work provides critical insights to guide the therapeutic development and scalable production of bacteriocins.

|
Scooped by
mhryu@live.com
Today, 11:05 AM
|
Non-genetic variability in gene expression is an inevitable consequence of the stochastic nature of processes driving transcription and translation. While previous studies demonstrated that gene expression noise is negatively correlated with gene body methylation, the function of this correlation remains poorly understood in multicellular systems. Here, we provide a first functional link between gene body methylation and transcription noise in plants. We investigated a mutant with partial loss of CG methylation (met1-1) and 10 epigenetic recombinant inbred lines (epiRILs) generated by a cross between Col-0 and met1-3 plants, and observed an increase in gene expression noise, but this was not the case in met1-3 with complete loss of CG methylation. Loss of CG methylation in met1-3 could be compensated by a low but significant gain of non-CG methylation that buffers the noise in gene expression. Overall, our results show that gene body methylation has a functional role in reducing variability in transcription in a large subset of housekeeping genes, which require precise expression patterns to meet metabolic requirements. Genes lacking this noise-buffering effect are mainly enriched in stress response, where variability in gene expression can be seen as highly beneficial.

|
Scooped by
mhryu@live.com
Today, 10:36 AM
|
Unconventional protein secretion (UcPS) enables the export of cytosolic proteins through pathways that bypass the canonical endoplasmic reticulum–Golgi secretory route. Although increasingly recognized as essential for intercellular communication, stress responses, and tissue homeostasis, UcPS remains difficult to quantify due to low secretion efficiency, high intracellular background, and the challenge of distinguishing active secretion from passive leakage. Recent methodological advances, including NanoLuc split luciferase–based reporters and the Retention Using Selective Hooks (RUSH) system for synchronized protein transport, have improved sensitivity and temporal control of trafficking. Here, we present complementary protocols integrating these tools to provide a highly sensitive, quantitative workflow centered on a split NanoLuc (HiBiT/LgBiT) complementation assay for monitoring UcPS in mammalian cells. The Basic Protocol describes a robust luminescence-based secretion assay, while the Support Protocols detail the generation of stable HiBiT reporter cell lines, approaches for probing UcPS mechanisms using siRNA-mediated gene knockdown and pharmacological perturbation, and the incorporation of the RUSH system to synchronize cargo release and identify potential trafficking intermediates. Together, these protocols provide a sensitive, scalable, high-throughput toolkit that enables analysis of UcPS mechanisms across diverse cargo proteins, cell types, and perturbations. This methodological framework allows for rigorous dissection of UcPS pathways in both physiological and disease-relevant contexts.

|
Scooped by
mhryu@live.com
Today, 10:28 AM
|
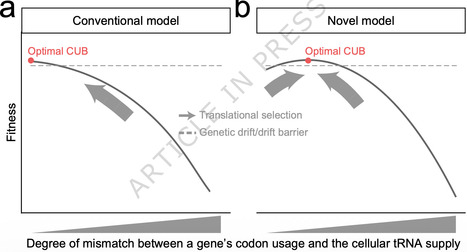
Each amino acid except two is encoded by multiple synonymous codons, but at unequal frequencies. Such codon usage bias (CUB) is observable in almost all species, and commonly assumed as the result of natural selection towards an optimal CUB that matches the cellular tRNA supply. Here we hypothesize instead that the optimal CUB of a gene should slightly mismatch the tRNA supply to avoid excessive translational costs, while ensuring adequate functional payoff. By modifying the CUB of a resistance gene expressed in bacteria under antibiotic selection, we demonstrate that a small mismatch with the tRNA supply confers faster bacterial growth than those with minimized or large CUB-tRNA mismatches. Intriguingly, the optimal degree of CUB-tRNA mismatch increases as the resistance gene becomes less important in media with lower antibiotic concentrations, which is explainable by our model as a shift in the balance between the gene’s functional payoff and translational cost. Furthermore, genomic analyses in model organisms suggest that the optimal degree of CUB-tRNA mismatch is larger for endogenous genes with lower functional importance and higher mRNA abundance, respectively supporting the impact of functional payoff and translational cost. Finally, we find that mutations increasing or decreasing the CUB-tRNA mismatch of native genes are both predominantly deleterious, such that the CUB-tRNA mismatch is likely selectively maintained rather than minimized to that achievable in the presence of genetic drift and mutational bias. These results challenge the commonly assumed unidirectional selection on CUB and highlight the CUB-modulated balance between functional payoff and translational cost. Nearly all organisms exhibit codon usage bias (CUB), traditionally thought to reflect a unidirectional selection maximally matching CUB and the cellular tRNA supply. Here, the authors propose alternatively that a slight mismatch optimizes the balance between translational cost and functional payoff.

|
Scooped by
mhryu@live.com
Today, 9:36 AM
|
3-Hydroxy-3-methylglutaryl-CoA synthase (HMGS) is a key enzyme in the mevalonate (MVA) pathway that catalyzes the formation of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) from acetoacetyl-CoA and acetyl-CoA. Recently, a novel class of HMGS-sesquiterpene synthase (STS) fusion enzymes has been identified. In this study, we discovered a natural fusion enzyme, GihirA, in Gloeostereum incarnatum, which contains both STS and HMGS domains and synthesizes the sesquiterpenoid hirsutene. Our investigation revealed that the HMGS domain significantly enhances the cyclization activity of the STS domain, resulting in an 8.87-fold increase in sesquiterpene production with a final yield of 121.3 mg/kg, highlighting HMGS’s critical role in catalytic efficiency. Additionally, domain-swapping experiments were performed by replacing the HMGS domain of G. incarnatum with the native HMGS domain from Flammulina velutipes sesquiterpene synthase Fla2. The results demonstrated that Fla2 fused with its cognate HMGS domain exhibited a significant yield enhancement from 11.5 to 54.5 mg/kg, underscoring the importance of metabolic compatibility in enzyme performance. This study not only reveals the unique advantages of natural fusion enzymes in sesquiterpene biosynthesis but also provides an important theoretical foundation for enhancing sesquiterpene production through the optimization of enzyme fusion strategies and metabolic pathway design. These findings offer a rational strategy for engineering high-efficiency terpenoid biosynthesis.

|
Scooped by
mhryu@live.com
Today, 1:31 AM
|
Gene duplication is the primary mechanism by which new genes emerge. Models and empirical studies have shown that paralogous genes are maintained because of dosage benefits, the partitioning of ancestral functions or the acquisition of new functions. However, the underlying molecular mechanisms and the relative importance of the factors driving evolution towards one fate or another have remained difficult to quantify. Recent advances in experimental and computational methods, such as gene editing, deep mutational scanning and ancestral sequence reconstruction, have enabled molecular analyses of duplicated gene evolution across timescales. Combined, these approaches are revealing how adaptive and non-adaptive evolutionary forces shape the modern fates of gene duplicates. Gene duplication is a key evolutionary mechanism, as initially redundant paralogues diverge over time. The authors review how adaptive and non-adaptive forces influence the evolutionary fates of gene duplicates, highlighting the importance of function–fitness relationships and gene expression dynamics.
|
 Your new post is loading...
Your new post is loading...



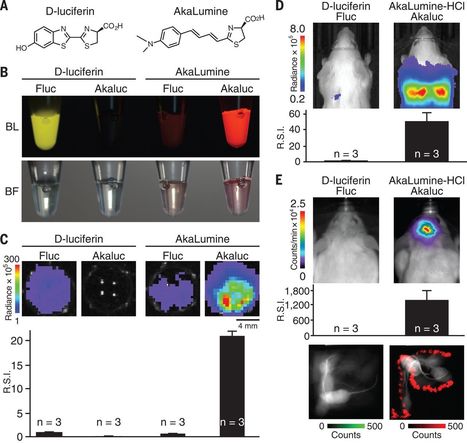


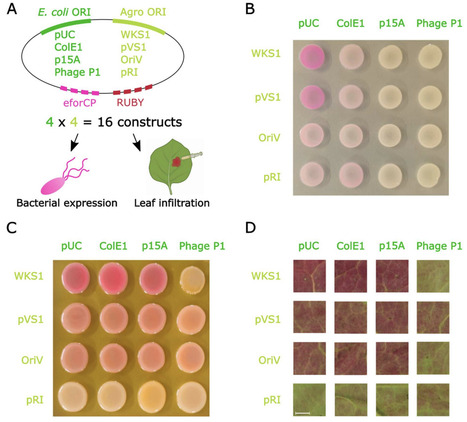
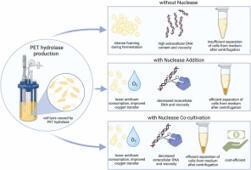
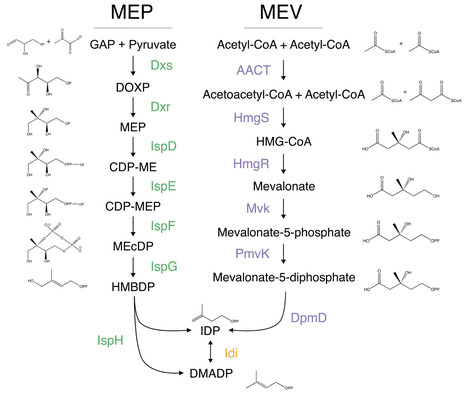

















wonder how this luciferase works in microbes and plants. What is the minimum conc. of AkaLumine (substrate) for luminescence?