Your new post is loading...
 Your new post is loading...

|
Scooped by
The Sainsbury Lab
July 16, 5:48 AM
|
Plants constantly monitor their environment to adapt to potential threats to their health and fitness. This involves cell-surface receptors that can detect conserved microbe-associated molecular patterns (MAMPs) or endogenous immunogenic signals, initiating signaling pathways to induce broad-spectrum disease resistance, known as pattern-triggered immunity (PTI). In Arabidopsis thaliana, the leucine-rich repeat receptor kinase (LRR-RK) MIK2 is an exceptionally versatile receptor involved in the perception of the vast family of Brassicales-specific endogenous SCOOP peptides as well as potential MAMPs derived from Fusarium and related fungi. Although only plant species belonging to the order of Brassicales encode genes for SCOOP peptides and show SCOOP-responsiveness, the Fusarium-derived elicitor fraction also induces PTI responses in plants from other lineages. In this study, we demonstrate that Fusarium elicitor-responsiveness and proteins belonging to the MIK2-clade are widely conserved among seed plants. We identified a MIK2-clade protein from tomato, which shares properties of AtMIK2 in the perception of the Fusarium elicitor but not of SCOOP peptides. Tomato mutants lacking the receptor show compromised PTI responses to the fungal elicitor and enhanced susceptibility to infection by Fusarium oxysporum. Our data provide insights into the evolutionary trajectory of MIK2 as a multifunctional receptor involved in plant immunity.

|
Scooped by
The Sainsbury Lab
July 16, 5:43 AM
|
Our usual encounters with fungi are when we observe mushrooms in forests or moulds on food that we failed to eat on time. In both instances, however, we are only seeing a very limited view of fungal growth. Fungi are osmotrophs, which means that they consume the food that surrounds them by secreting enzymes to degrade polymers into simple sugars, fatty acids and amino acids. This type of growth has a number of consequences - it explains why fungi secrete toxins and antibiotics to protect their food sources from competitors and why they can grow so rapidly. It also explains why fungi have evolved the capacity to forcefully invade hard substrates, such as wood, animal skins and the cuticles of plants. By doing so, they can access new sources of food, often inaccessible to their competition, and this has enabled fungi to become highly successful pathogens of both animals and plants, causing diseases in organisms as diverse as insects, amphibians, humans, reptiles, and rice plants. It is becoming clear that, in addition to their prodigious secretion of enzymes to degrade complex substrates, fungi can exert very substantial physical forces. Such forces are probably essential for many aspects of the fungal lifestyle, including colonisation of their usual habitats like soil and leaf litter, which require penetration and invasion to enable their digestion by fungi. But for pathogenic fungi, the requirement for invasive growth is even more acute. In this Primer, we explore the mechanobiology of fungal invasive growth and the emerging view of the different mechanisms that fungal pathogens deploy to gain entry to host tissue. We focus mainly on plant pathogens, where recent experimental work has been most extensive, and highlight key research questions for the future.

|
Scooped by
The Sainsbury Lab
July 16, 5:36 AM
|
Plant nucleotide-binding, leucine-rich repeat (NLR) immune receptors recognize pathogen effectors and activate defense. NLR genes can be non-functiona…

|
Scooped by
The Sainsbury Lab
July 16, 5:08 AM
|
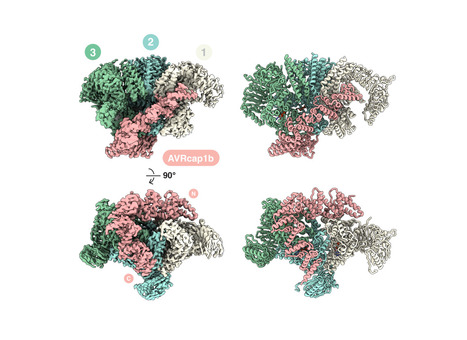
Helper NLRs function as central nodes in plant immune networks. Upon activation, they oligomerize into inflammasome like resistosomes to initiate immune signaling, yet the dynamics of resistosome assembly remain poorly understood. Here, we show that the virulence effector AVRcap1b from the Irish potato famine pathogen Phytophthora infestans suppresses immune activation by directly engaging oligomerization intermediates of the tomato helper NLR SlNRC3. CryoEM structures of SlNRC3 in AVRcap1b bound and unbound states reveal that AVRcap1b bridges multiple protomers, stabilizing a stalled intermediate and preventing formation of a functional resistosome. Leveraging AVRcap1b as a molecular tool, we also capture an additional SlNRC3 resistosome intermediate showing that assembly proceeds in a stepwise manner from dissociated monomers. These findings uncover a previously unrecognized vulnerability in NLR activation and reveal a pathogen strategy that disrupts immune complex assembly. This work advances mechanistic understanding of resistosome formation and uncovers a previously unrecognized facet of pathogen-plant coevolution.

|
Scooped by
The Sainsbury Lab
April 28, 3:41 PM
|
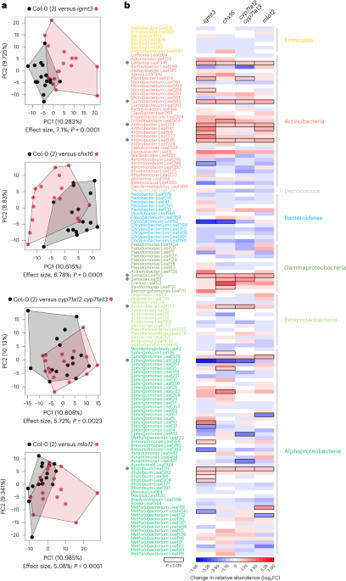
Key message Plant U-box E3 ligases PUB20 and PUB21 are flg22-triggered signaling components and negatively regulate immune responses. Abstract Plant U-box proteins (PUBs) constitute a class of E3 ligases that are associated with various stress responses. Among the class IV PUBs featuring C-terminal Armadillo (ARM) repeats, PUB20 and PUB21 are closely related homologs. Here, we show that both PUB20 and PUB21 negatively regulate innate immunity in plants. Loss of PUB20 and PUB21 function leads to enhanced resistance to surface inoculation with the virulent bacterium Pseudomonas syringae pv. tomato DC3000 (Pst DC3000). However, the resistance levels remain unaffected after infiltration inoculation, suggesting that PUB20 and PUB21 primarily function during the early defense stages. The enhanced resistance to Pst DC3000 in PUB mutant plants (pub20-1, pub21-1, and pub20-1/pub21-1) correlates with extensive flg22-triggered reactive oxygen production, strong MPK3 activation, and enhanced transcriptional activation of early immune response genes. Additionally, PUB mutant plants (except pub21-1) exhibit constitutive stomatal closure after Pst DC3000 inoculation, implying the significant role of PUB20 in stomatal immunity. Comparative analyses of flg22 responses between PUB mutants and wild-type plants reveals that the robust activation of the pattern-induced immune responses may enhance resistance against Pst DC3000. Notably, the hypersensitivity responses triggered by RPM1/avrRpm1 and RPS2/avrRpt2 are independent of PUB20 and PUB21. These results suggest that PUB20 and PUB21 knockout mutations affect bacterial invasion, likely during the early stages, acting as negative regulators of plant immunity.

|
Scooped by
The Sainsbury Lab
April 28, 3:12 PM
|
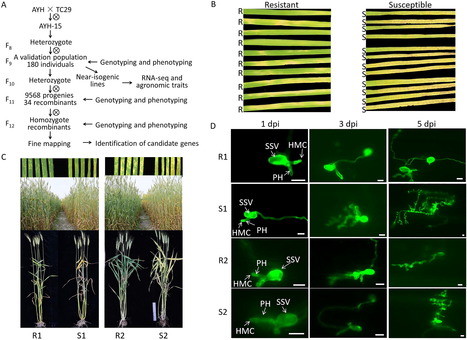
Stripe rust, caused by Puccinia striiformis f. sp. tritici ( Pst), is a devastating disease in wheat worldwide. Discovering and characterizing new resistance genes/QTL is crucial for wheat breeding programs. In this study, we fine-mapped and characterized a stripe rust resistance gene, YRAYH, on chromosome arm 5BL in the Chinese wheat landrace Anyuehong (AYH). Evaluations of stripe rust response to prevalent Chinese Pst races in near-isogenic lines derived from a cross of Anyuehong and Taichung 29 showed that YrAYH conferred a high level of resistance at all growth stages. Fine mapping using a large segregating population of 9748 plants, narrowed the YRAYH locus to a 3.7 Mb interval on chromosome arm 5BL that included 61 annotated genes. Transcriptome analysis of two NIL pairs identified 64 upregulated differentially expressed genes (DEGs) in the resistant NILs (NILs-R). Annotations indicated that many of these genes have roles in plant disease resistance pathways. Through a combined approach of fine-mapping and transcriptome sequencing, we identified a serine/threonine-protein kinase SRPK as a candidate gene underlying YrAYH. A unique 25 bp insertion was identified in the NILs-R compared to the NILs-S and previously published wheat genomes. An InDel marker was developed and co-segregated with YrAYH. Agronomic trait evaluation of the NILs suggested that YrAYH not only reduces the impact of stripe rust but was also associated with a gene that increases plant height and spike length.

|
Scooped by
The Sainsbury Lab
February 12, 5:01 AM
|
-
Asian soybean rust (ASR), caused by the obligate biotrophic fungus, Phakopsora pachyrhizi, was first reported in the continental United States of America (USA) in 2004 and over the years has been of concern to soybean production in the USA. The prevailing hypothesis is that P. pachyrhizi spores were introduced into the USA via hurricanes originating from South America, particularly Hurricane Ivan. -
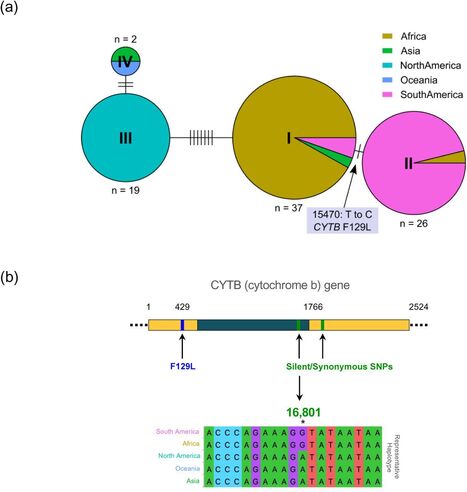
To investigate the genetic diversity and global population structure of P. pachyrhizi, we employed exome-capture based sequencing on 84 field isolates collected from different geographic regions worldwide. We compared the gene-encoding regions from all these field isolates and found that four major haplotypes are prevalent worldwide. Here, we provide genetic evidence supporting multiple incursions that have led to the currently established P. pachyrhizi population of the USA. Phylogenetic analysis of mitochondrial genes further supports this hypothesis. -
Notably, we observed limited genetic diversity in P. pachyrhizi populations in Brazil, suggesting a clonal population structure in that country that contrasts to populations from the USA and Africa. -
This study provides the first comprehensive characterization of P. pachyrhizi population structures defined by genetic evidence from populations across major soybean growing regions.

|
Scooped by
The Sainsbury Lab
February 12, 4:43 AM
|
Carbohydrate-based cell wall signaling impacts plant growth, development, and stress responses; however, how cell wall signals are perceived and transduced remains poorly understood. Several cell wall breakdown products have been described as typical damage-associated molecular patterns that activate plant immunity, including pectin-derived oligogalacturonides (OGs). Receptor kinases of the WALL-ASSOCIATED KINASE (WAK) family bind pectin and OGs and were previously proposed as OG receptors. However, unambiguous genetic evidence for the role of WAKs in OG responses is lacking. Here, we investigated the role of Arabidopsis (Arabidopsis thaliana) WAKs in OG perception using a clustered regularly interspaced short palindromic repeats mutant in which all 5 WAK genes were deleted. Using a combination of immune assays for early and late pattern-triggered immunity, we show that WAKs are dispensable for OG-induced signaling and immunity, indicating that they are not bona fide OG receptors.

|
Scooped by
The Sainsbury Lab
February 12, 4:40 AM
|
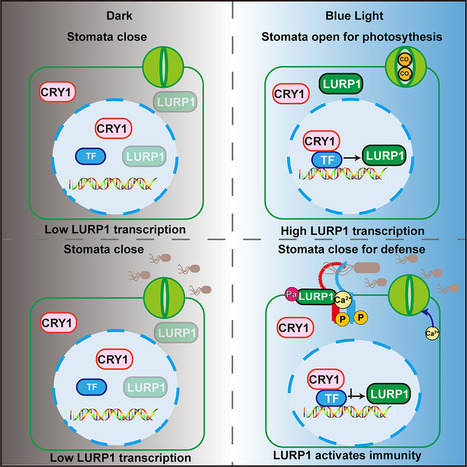
Plant stomata open in response to blue light, allowing gas exchange and water transpiration. However, open stomata are potential entry points for pathogens. Whether plants can sense pathogens and mount defense responses upon stomatal opening and how blue-light cues are integrated to balance growth-defense trade-offs are poorly characterized. We show that the Arabidopsis blue-light photoreceptor CRYPTOCHROME 1 (CRY1) mediates various aspects of immunity, including pathogen-triggered stomatal closure as well as activation of plant immunity through a typical light-responsive protein LATE UPREGULATED IN RESPONSE TO HYALOPERONOSPORA PARASITICA (LURP1). LURP1 undergoes N-terminal palmitoylation in the presence of bacterial flagellin, prompting a change in subcellular localization from the cytoplasm to plasma membrane, where it enhances the activity of the receptor FLAGELLIN SENSING 2 (FLS2) to mediate plant defense. Collectively, these findings reveal that blue light regulates stomatal defense and highlight the dual functions of CRY1 in photosynthesis and immunity.

|
Scooped by
The Sainsbury Lab
February 12, 4:31 AM
|
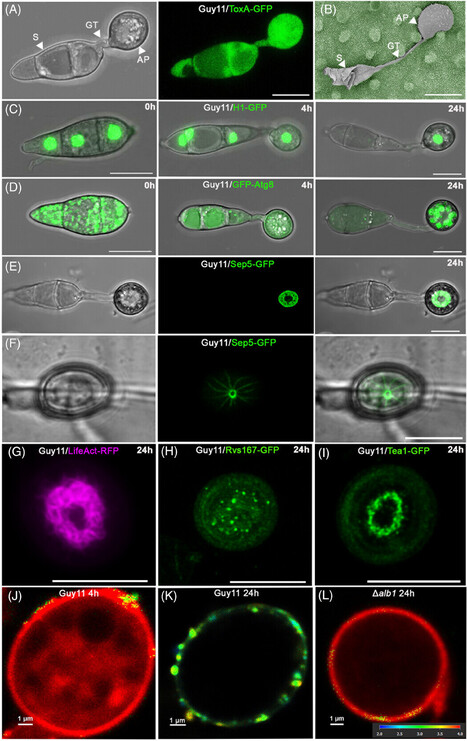
Magnaporthe oryzae is the causal agent of rice blast, one of the most serious diseases affecting rice cultivation around the world. During plant infection, M. oryzae forms a specialised infection structure called an appressorium. The appressorium forms in response to the hydrophobic leaf surface and relies on multiple signalling pathways, including a MAP kinase phosphorelay and cAMP-dependent signalling, integrated with cell cycle control and autophagic cell death of the conidium. Together, these pathways regulate appressorium morphogenesis.The appressorium generates enormous turgor, applied as mechanical force to breach the rice cuticle. Re-polarisation of the appressorium requires a turgor-dependent sensor kinase which senses when a critical threshold of turgor has been reached to initiate septin-dependent re-polarisation of the appressorium and plant infection. Invasive growth then requires differential expression and secretion of a large repertoire of effector proteins secreted by distinct secretory pathways depending on their destination, which is also governed by codon usage and tRNA thiolation. Cytoplasmic effectors require an unconventional Golgi-independent secretory pathway and evidence suggests that clathrin-mediated endocytosis is necessary for their delivery into plant cells. The blast fungus then develops a transpressorium, a specific invasion structure used to move from cell-to-cell using pit field sites containing plasmodesmata, to facilitate its spread in plant tissue. This is controlled by the same MAP kinase signalling pathway as appressorium development and requires septin-dependent hyphal constriction. Recent progress in understanding the mechanisms of rice infection by this devastating pathogen using live cell imaging procedures are presented.

|
Scooped by
The Sainsbury Lab
February 11, 3:44 PM
|
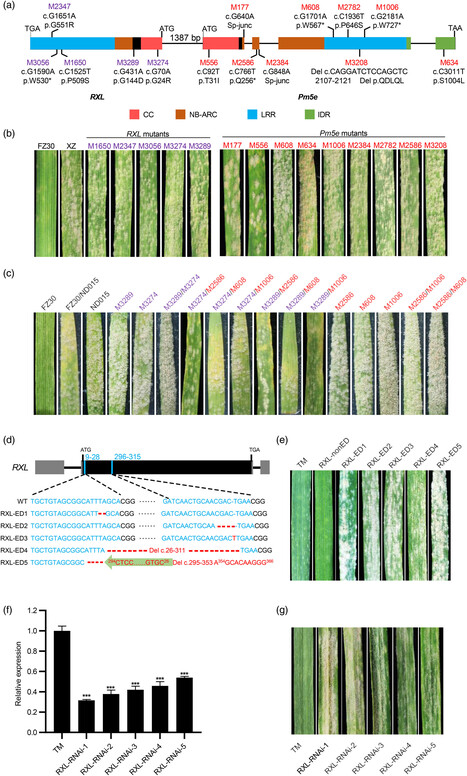
Powdery mildew poses a significant threat to global wheat production and most cloned and deployed resistance genes for wheat breeding encode nucleotide-binding and leucine-rich repeat (NLR) immune receptors. Although two genetically linked NLRs function together as an NLR pair have been reported in other species, this phenomenon has been relatively less studied in wheat. Here, we demonstrate that two tightly linked NLR genes, RXL and Pm5e, arranged in a head-to-head orientation, function together as an NLR pair to mediate powdery mildew resistance in wheat. The resistance function of the RXL/Pm5e pair is validated by mutagenesis, gene silencing, and gene-editing assays. Interestingly, both RXL and Pm5e encode atypical NLRs, with RXL possessing a truncated NB-ARC (nucleotide binding adaptor shared by APAF-1, plant R proteins and CED-4) domain and Pm5e featuring an atypical coiled-coil (CC) domain. Notably, RXL and Pm5e lack an integrated domain associated with effector recognition found in all previously reported NLR pairs. Additionally, RXL and Pm5e exhibit a preference for forming hetero-complexes rather than homo-complexes, highlighting their cooperative role in disease resistance. We further show that the CC domain of Pm5e specifically suppresses the hypersensitive response induced by the CC domain of RXL through competitive interaction, revealing regulatory mechanisms within this NLR pair. Our study sheds light on the molecular mechanism underlying RXL/Pm5e-mediated powdery mildew resistance and provides a new example of an NLR pair in wheat disease resistance.

|
Scooped by
The Sainsbury Lab
February 11, 3:24 PM
|
Soybean [Glycine max (L.) Merrill] is one of the most widely grown legumes in the world, with Brazil being its largest producer and exporter. Breeding programs in Brazil have resulted from multiple cycles of selection and recombination starting from a small number of USA cultivar ancestors in the 1950s and 1960s years. This process has led to the successful adaptation of this crop to tropical conditions, a phenomenon known as tropicalization. Many studies describe a narrow genetic background in Brazilian soybean cultivars. Various factors can affect the genetic diversity in species, especially in cultivated crops, such as the reproduction type, artificial selection, and the number and sources of variability in the breeding programs. In turns, the genetic diversity can affect the linkage disequilibrium blocks (LD) patterns and, consequently, molecular breeding strategies for selection of target loci for agronomic traits. We used high-throughput genotyping with SoySNP50K Illumina SNP markers to assess a collection of 370 Brazilian soybean accessions covering more than 60 years of soybean breeding in Brazil. Our goal was to investigate population structure and genetic diversity in the Brazilian germplasm, detect patterns of LD blocks, and identify regions presenting signals of selective swaps linked with quantitative trait loci (QTLs) of agronomic interest. Population structure analysis revealed two major groups among all genotypes, primarily differentiated by the year of release, separating old and new cultivars (before and after 2000´s years), and by growth habit (stem termination type—SST). The group I comprises about 75% of the panel and includes cultivars release before 2000`s years, including the oldest cultivars released in Brazil, most of which exhibit a determinate growth habit and maturity groups VI and VII. Group II includes only 83 materials, but shows higher levels of diversity than group I, representing most recent introductions in Brazilian germplasm. Further analysis of substructure within Group I, identified seven subgroups with no clear trend for segregation based on maturity group, STT or year of release. Instead, these subgroups were based on the contribution of key donors of disease resistance and adaptability, as soybean cultivation expanded from the South to Central region of Brazil. This finding is consistent with the history of soybean expansion in Brazil. We identified 123 genomic regions under selection among the groups of Brazilian cultivars associated with 440 quantitative trait loci (QTLs), revealing regions fixed across the breeding process associated with yield, disease resistance, water efficiency use, and others.

|
Scooped by
The Sainsbury Lab
December 12, 2024 12:01 PM
|
Plants have evolved an elaborate cell wall integrity (CWI) sensing system to monitor and modify cell wall formation. LRR-extensins (LRXs) are cell wall-anchored proteins that bind RAPID ALKALINIZATION FACTOR (RALF) peptide hormones and induce compaction of cell wall structures. At the same time, LRXs form a signaling platform with RALFs and the transmembrane receptor kinase FERONIA (FER) as a means to relay changes in CWI to the protoplast. LRX1 of Arabidopsis thaliana is predominantly expressed in root hairs and lrx1mutants develop defective root hairs. Here, we identify a regulator of LRX1-RALF-FER signaling as a suppressor of the lrx1 root hair phenotype. The repressor of lrx1_23 (rol23) gene encodes PP2C12, a type 2C phosphatase of clade H that interacts with FER and dephosphorylates Thr696 in the FER activation loop in vitro. The LRX1-related function of PP2Cs appears clade H-specific and was not observed for other PP2Cs investigated. Collectively, our data suggest that LRX1 acts upstream of the RALF1-FER signaling module and PP2C12 has an inhibitory activity via modulating FER activity to fine-tune CWI signaling.
|

|
Scooped by
The Sainsbury Lab
July 16, 5:46 AM
|
The receptor-like cytoplasmic kinase BIK1 and its close homolog PBL1 have been widely recognized as central components of plant immunity. However, most genetic studies of BIK1 and PBL1 functions were carried out with single T-DNA insertional mutant alleles. Some phenotypes observed in these mutants, e.g. autoimmunity, have been difficult to reconcile with the proposed role of BIK1 and PBL1 in pattern-triggered immunity. In this study, we generated multiple new alleles of bik1 and pbl1 by CRISPR-Cas9-based gene editing and systematically analyzed these mutants alongside existing T-DNA insertional lines. These analyses reinforced the central role of BIK1 and PBL1 in pattern-triggered immunity mediated by both receptor kinases and receptor-like proteins. At the same time, however, we revealed several pleiotropic phenotypes associated with T-DNA insertions that are not necessarily linked to loss of BIK1 or PBL1 function. Further analyses of newly generated bik1 pbl1 double mutants uncovered an even greater contribution of these kinases to immune signaling and disease resistance than previously appreciated. These findings clarify longstanding ambiguities surrounding BIK1 and PBL1 functions.

|
Scooped by
The Sainsbury Lab
July 16, 5:41 AM
|
Plant nucleotide-binding, leucine-rich-repeat (NLR) immune receptors recognize pathogen effectors and activate immunity. The NLR RPS2 recognizes AvrRpt2, a Pseudomonas effector that promotes virulence by proteolytically cleaving a membrane-tethered host protein, RIN4. RIN4 cleavage by AvrRpt2 generates fragments that activate RPS2. A model for RPS2 activation by RIN4 destruction is consistent with the ectopic activity of RPS2 in plants lacking RIN4 but does not explain the link between AvrRpt2’s virulence activity and RPS2 activation. We found that non-membrane-tethered RIN4 derivatives are potent cytosolic activators of RPS2. Activation of RPS2 by these RIN4 derivatives, like AvrRpt2-induced activation, and unlike ectopic activation in the absence of RIN4, requires the defense signaling protein NDR1. Cleavage products of RIN4 produced by AvrRpt2 play contrasting roles in the activation of RPS2, with the membrane-tethered C-terminal fragment suppressing RPS2 and the non-membrane-tethered internal fragment, dependent on compatibility with the C-terminal fragment, overcoming its suppression of RPS2.

|
Scooped by
The Sainsbury Lab
July 16, 5:32 AM
|
Introducing and characterizing variation through mutagenesis plus functional genomics can accelerate resistance breeding as well as our understanding of crop plant immunity. To reveal new germplasm resources for fungal disease resistance breeding in elite durum wheat, we challenged the diverse alleles in a sequenced and cataloged ethyl methanesulfonate mutagenized population of elite tetraploid wheat Triticum turgidum subsp. durum cv ‘Kronos’ with stripe rust. We screened 2,000 mutant lines and identified sixteen enhanced disease resistance (EDR) lines with persistent resistance to stripe rust over four years of field testing. To find broad-spectrum resistance, we challenged these lines with other major biotrophic and necrotrophic pathogens, including those causing Septoria tritici blotch, tan spot, Fusarium head blight and leaf rust. Enhanced resistance to multiple fungi was found in 13 of 16 EDR lines. Five EDR lines showed spontaneous lesion formation in the absence of pathogens, providing new mutant resources to study plant stress response in the absence of the confounding effects of pathogen infection. We mapped exome capture sequencing data of the EDR lines to a recently released long-read Kronos genome to aid in the identification of causal mutations. We located an EDR resistance locus to an 175 Mb interval on chromosome 1B. Importantly, these phenotypically characterized EDR lines are newly described durum germplasm coupled with improved functional genomics resources that are readily available for both wheat fungal resistance breeding and basic plant immunity research.

|
Scooped by
The Sainsbury Lab
July 7, 11:43 AM
|
Plant nucleotide-binding domain and leucine-rich repeat immune receptors (NLRs) confer disease resistance to many foliar and root parasites. However, the extent to which NLR-mediated immunity is differentially regulated between plant organs is poorly known. Here, we show that a large cluster of tomato (Solanum lycopersicum) genes, encoding the cyst and root-knot nematode disease resistance proteins Hero and MeR1 as well as the NLR helper NLR required for cell death 6 (NRC6), is nearly exclusively expressed in the roots. This root-specific gene cluster emerged in Solanum species about 21 million years ago through gene duplication of the ancient asterid NRC network. NLR sensors in this gene cluster function exclusively through NRC6 helpers to trigger hypersensitive cell death. These findings indicate that the NRC6 gene cluster has sub-functionalized from the larger NRC network to specialize in mediating resistance against root pathogens, including cyst and root-knot nematodes. We propose that some NLR gene clusters and networks may have evolved organ-specific gene expression as an adaptation to particular parasites and to reduce the risk of autoimmunity.

|
Scooped by
The Sainsbury Lab
April 28, 3:26 PM
|
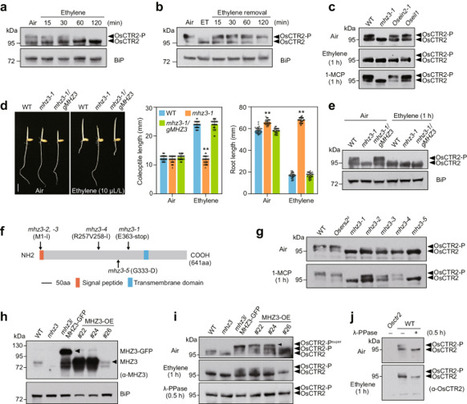
Ethylene regulates plant growth, development, and stress adaptation. However, the early signaling events following ethylene perception, particularly in the regulation of ethylene receptor/CTRs (CONSTITUTIVE TRIPLE RESPONSE) complex, remains less understood. Here, utilizing the rapid phospho-shift of rice OsCTR2 in response to ethylene as a sensitive readout for signal activation, we revealed that MHZ3, previously identified as a stabilizer of ETHYLENE INSENSITIVE 2 (OsEIN2), is crucial for maintaining OsCTR2 phosphorylation. Genetically, both functional MHZ3 and ethylene receptors prove essential for OsCTR2 phosphorylation. MHZ3 physically interacts with both subfamily I and II ethylene receptors, e.g., OsERS2 and OsETR2 respectively, stabilizing their association with OsCTR2 and thereby maintaining OsCTR2 activity. Ethylene treatment disrupts the interactions within the protein complex MHZ3/receptors/OsCTR2, reducing OsCTR2 phosphorylation and initiating downstream signaling. Our study unveils the dual role of MHZ3 in fine-tuning ethylene signaling activation, providing insights into the initial stages of the ethylene signaling cascade. The early signalling events following ethylene perception by plants remain incompletely understood. Here the authors show that in the absence of ethylene, rice MHZ3, a known stabilizer of OsEIN2, promotes phosphorylation of OsCTR2 to suppress ethylene signalling.

|
Scooped by
The Sainsbury Lab
February 12, 5:09 AM
|
-
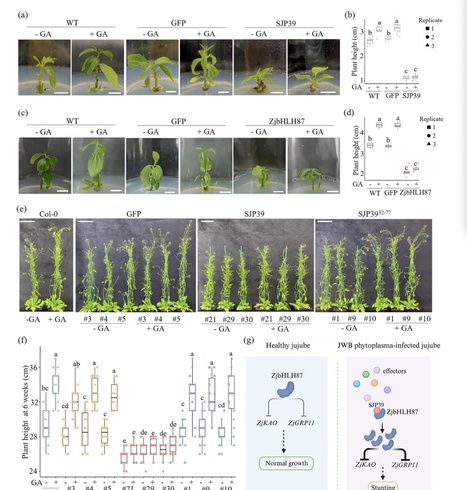
Phytoplasmas are specialized phloem-limited bacteria that cause diseases on various crops resulting in significant agricultural losses. This research focuses on the jujube Witches’ Broom (JWB) phytoplasma and investigates the host-manipulating activity of the effector SJP39. -
We found that SJP39 directly interacts with the plant transcription factor bHLH87 in the nuclei. SJP39 stabilizes the bHLH87 homologs in A. thaliana and jujube, leading to growth defects in the plants. -
Transcriptomic analysis indicates that SJP39 affects the gibberellin (GA) pathway in jujube. We further demonstrate that ZjbHLH87 regulates GA signalling as a negative regulator and SJP39 enhances this regulation. -
The research offers important insights into the pathogenesis of JWB disease and identified SJP39 as a virulence factor that can contribute to the growth defects caused by JWB phytoplasma infection. These findings open new opportunities to manage JWB and other phytoplasma diseases.

|
Scooped by
The Sainsbury Lab
February 12, 4:48 AM
|
The ability of plants to perceive and react to biotic and abiotic stresses is critical for their health. We recently identified a core set of genes consistently induced by members of the leaf microbiota, termed general non-self response (GNSR) genes. Here we show that GNSR components conversely impact leaf microbiota composition. Specific strains that benefited from this altered assembly triggered strong plant responses, suggesting that the GNSR is a dynamic system that modulates colonization by certain strains. Examination of the GNSR to live and inactivated bacteria revealed that bacterial abundance, cellular composition and exposure time collectively determine the extent of the host response. We link the GNSR to pattern-triggered immunity, as diverse microbe- or danger-associated molecular patterns cause dynamic GNSR gene expression. Our findings suggest that the GNSR is the result of a dose-responsive perception and signalling system that feeds back to the leaf microbiota and contributes to the intricate balance of plant–microbiome interactions. The plant general non-self response system is triggered by leaf microbiota members and, in turn, impacts their colonization.

|
Scooped by
The Sainsbury Lab
February 12, 4:41 AM
|
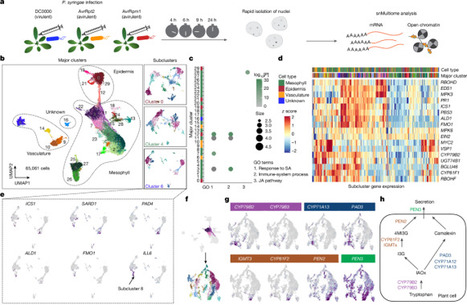
Plants lack specialized and mobile immune cells. Consequently, any cell type that encounters pathogens must mount immune responses and communicate with surrounding cells for successful defence. However, the diversity, spatial organization and function of cellular immune states in pathogen-infected plants are poorly understood1. Here we infect Arabidopsis thaliana leaves with bacterial pathogens that trigger or supress immune responses and integrate time-resolved single-cell transcriptomic, epigenomic and spatial transcriptomic data to identify cell states. We describe cell-state-specific gene-regulatory logic that involves transcription factors, putative cis-regulatory elements and target genes associated with disease and immunity. We show that a rare cell population emerges at the nexus of immune-active hotspots, which we designate as primary immune responder (PRIMER) cells. PRIMER cells have non-canonical immune signatures, exemplified by the expression and genome accessibility of a previously uncharacterized transcription factor, GT-3A, which contributes to plant immunity against bacterial pathogens. PRIMER cells are surrounded by another cell state (bystander) that activates genes for long-distance cell-to-cell immune signalling. Together, our findings suggest that interactions between these cell states propagate immune responses across the leaf. Our molecularly defined single-cell spatiotemporal atlas provides functional and regulatory insights into immune cell states in plants. The development of a molecularly defined spatiotemporal atlas of pathogen-infected Arabidopsis thaliana leaves reveals specific cell states that have distinct roles in plant immunity.

|
Scooped by
The Sainsbury Lab
February 12, 4:36 AM
|
Fungi are the most important group of plant pathogens, responsible for many of the world’s most devastating crop diseases. One of the reasons they are such successful pathogens is because several fungi have evolved the capacity to breach the tough outer cuticle of plants using specialized infection structures called appressoria. This is exemplified by the filamentous ascomycete fungus Magnaporthe oryzae, causal agent of rice blast, one of the most serious diseases affecting rice cultivation globally. M. oryzae develops a pressurized dome-shaped appressorium that uses mechanical force to rupture the rice leaf cuticle. Appressoria form in response to the hydrophobic leaf surface, which requires the Pmk1 MAP kinase signalling pathway, coupled to a series of cell-cycle checkpoints that are necessary for regulated cell death of the fungal conidium and development of a functionally competent appressorium. Conidial cell death requires autophagy, which occurs within each cell of the spore, and is regulated by components of the cargo-independent autophagy pathway. This results in trafficking of the contents of all three cells to the incipient appressorium, which develops enormous turgor of up to 8.0 MPa, due to glycerol accumulation, and differentiates a thickened, melanin-lined cell wall. The appressorium then re-polarizes, re-orienting the actin and microtubule cytoskeleton to enable development of a penetration peg in a perpendicular orientation, that ruptures the leaf surface using mechanical force. Re-polarization requires septin GTPases which form a ring structure at the base of the appressorium, which delineates the point of plant infection, and acts as a scaffold for actin re-localization, enhances cortical rigidity, and forms a lateral diffusion barrier to focus polarity determinants that regulate penetration peg formation. Here we review the mechanism of regulated cell death in M. oryzae, which requires autophagy but may also involve ferroptosis. We critically evaluate the role of regulated cell death in appressorium morphogenesis and examine how it is initiated and regulated, both temporally and spatially, during plant infection. We then use this synopsis to present a testable model for control of regulated cell death during appressorium-dependent plant infection by the blast fungus.

|
Scooped by
The Sainsbury Lab
February 11, 3:46 PM
|
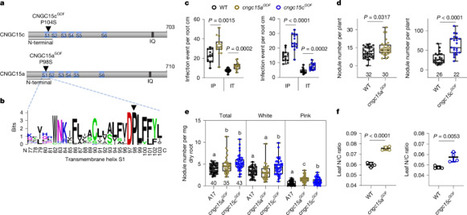
Nutrient acquisition is crucial for sustaining life. Plants develop beneficial intracellular partnerships with arbuscular mycorrhiza (AM) and nitrogen-fixing bacteria to surmount the scarcity of soil nutrients and tap into atmospheric dinitrogen, respectively1,2. Initiation of these root endosymbioses requires symbiont-induced oscillations in nuclear calcium (Ca2+) concentrations in root cells3. How the nuclear-localized ion channels, cyclic nucleotide-gated channel (CNGC) 15 and DOESN’T MAKE INFECTIONS1 (DMI1)4 are coordinated to specify symbiotic-induced nuclear Ca2+ oscillations remains unknown. Here we discovered an autoactive CNGC15 mutant that generates spontaneous low-frequency Ca2+ oscillations. While CNGC15 produces nuclear Ca2+ oscillations via a gating mechanism involving its helix 1, DMI1 acts as a pacemaker to specify the frequency of the oscillations. We demonstrate that the specificity of symbiotic-induced nuclear Ca2+ oscillations is encoded in its frequency. A high frequency activates endosymbiosis programmes, whereas a low frequency modulates phenylpropanoid pathways. Consequently, the autoactive cngc15 mutant, which is capable of generating both frequencies, has increased flavonoids that enhance AM, root nodule symbiosis and nutrient acquisition. We transferred this trait to wheat, resulting in field-grown wheat with increased AM colonization and nutrient acquisition. Our findings reveal a new strategy to boost endosymbiosis in the field and reduce inorganic fertilizer use while sustaining plant growth. Nuclear calcium oscillations initiate plant–arbuscular mycorrhiza and nitrogen-fixing bacteria symbioses for nutrient acquisition, with a newly discovered autoactive CNGC15 mutant enhancing these partnerships, potentially improving crop nutrition and reducing inorganic fertilizer dependence.

|
Scooped by
The Sainsbury Lab
February 11, 3:30 PM
|
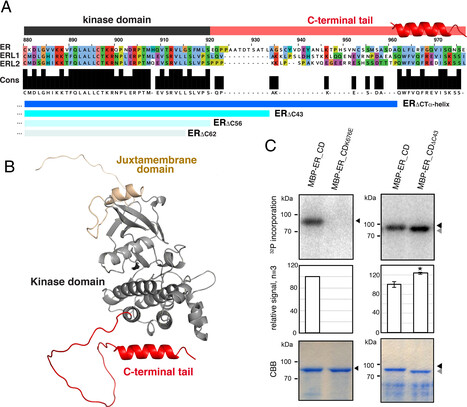
Cells perceive and process external signals through their cell-surface receptors, whose activity must be tightly maintained to prevent the spread of misinformation. How do plant cells prevent inappropriate receptor activity? We identify a structural module within the C-terminal tail of the receptor-kinase ERECTA (ER_CT) that inhibits the receptor pre- and post-signal activation. The ER_CT comprises a linker and an α-Helix. Before activation, ER_CT is autoinhibitory and associates with an inhibitory protein. Ligand perception triggers the transphosphorylation of ER_CT by the coreceptor, which then recruits a degradation machinery to turn over the activated receptor swiftly. Thus, we reveal an off–on–off toggle switch mechanism that finely adjusts the activity of the plant receptor, enabling precise control over cell signaling.

|
Scooped by
The Sainsbury Lab
February 11, 3:21 PM
|
Lichens are composite, symbiotic associations of fungi, algae, and bacteria that result in large, anatomically complex organisms adapted to many of the world’s most challenging environments. How such intricate, self-replicating lichen architectures develop from simple microbial components remains unknown because of their recalcitrance to experimental manipulation. Here, we report a metagenomic and metatranscriptomic analysis of the lichen Xanthoria parietina at different developmental stages. We identified 168 genomes of symbionts and lichen-associated microbes across the sampled thalli, including representatives of green algae, three different classes of fungi, and 14 bacterial phyla. By analyzing the occurrence of individual species across lichen thalli from diverse environments, we defined both substrate-specific and core microbial components of the lichen. Metatranscriptomic analysis of the principal fungal symbiont from three different developmental stages of a lichen, compared with axenically grown fungus, revealed differential gene expression profiles indicative of lichen-specific transporter functions, specific cell signaling, transcriptional regulation, and secondary metabolic capacity. Putative immunity-related proteins and lichen-specific structurally conserved secreted proteins resembling fungal pathogen effectors were also identified, consistent with a role for immunity modulation in lichen morphogenesis.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...