Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
June 4, 2021 1:41 PM
|
Resistance represents a major challenge for antibody-based therapy for coronavirus disease 2019 (COVID-19)1–4. Here we engineered an immunoglobulin M (IgM) neutralizing antibody (IgM-14) to overcome the resistance encountered by IgG-based therapeutics. IgM-14 is >230-fold more potent than its parental IgG-14 in neutralizing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). IgM-14 potently neutralizes the resistant virus raised by its corresponding IgG-14, the newly emerged United Kingdom B.1.1.7, Brazilian P.1, and South African B.1.351 variants of concern (VOCs), and 21 other receptor-binding domain (RBD) mutants, many of which are resistant to the IgGs that have been authorized for emergency use. Although engineering IgG into IgM enhances antibody potency in general, selection of an optimal epitope is critical for identifying the most effective IgM that can overcome resistance. One single intranasal (IN) dose of 0.044 and 0.4 mg/kg IgM-14 confers prophylactic and therapeutic efficacy against SARS-CoV-2 in mice, respectively. IgM-14, but not IgG-14, also confers potent therapeutic protection against the P.1 and B.1.351 variants. IgM-14 exhibits desirable IN pharmacokinetics and safety in rodents. Our results demonstrate that IN administration of an engineered IgM can improve efficacy, reduce resistance, and simplify the prophylactic and therapeutic treatment of COVID-19.

|
Scooped by
Gilbert C FAURE
May 19, 2021 1:52 PM
|
New technologies are expected to enhance nasal vaccines industry growth as particular attention is being paid to designing delivery strategies that take into account the broad range of diseases, populations and healthcare delivery settings that will benefit from the mucosal route of delivery.

|
Scooped by
Gilbert C FAURE
May 19, 2021 1:48 PM
|
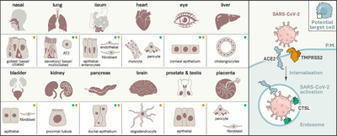
Analyses from single-cell sequencing datasets support the idea that COVID-19 is not just a respiratory disease but an illness that can affect multiple organs.

|
Scooped by
Gilbert C FAURE
May 7, 2021 3:26 AM
|
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan City, China, in 2019. After that, the outbreak has grown into a global pandemic and definite treatment for the disease, termed coronavirus disease 2019 (COVID-19), is currently unavailable.

|
Scooped by
Gilbert C FAURE
April 29, 2021 10:12 AM
|
COVID-19, caused by SARS-CoV-2, can result in acute respiratory distress syndrome and multiple-organ failure1–4, but little is known about its pathophysiology. Here, we generated single-cell atlases of 23 lung, 16 kidney, 16 liver and 19 heart COVID-19 autopsy donor tissue samples, and spatial atlases of 14 lung donors. Integrated computational analysis uncovered substantial remodeling in the lung epithelial, immune and stromal compartments, with evidence of multiple paths of failed tissue regeneration, including defective alveolar type 2 differentiation and expansion of fibroblasts and putative TP63+ intrapulmonary basal-like progenitor cells. Viral RNAs were enriched in mononuclear phagocytic and endothelial lung cells which induced specific host programs. Spatial analysis in lung distinguished inflammatory host responses in lung regions with and without viral RNA. Analysis of the other tissue atlases showed transcriptional alterations in multiple cell types in COVID-19 donor heart tissue, and mapped cell types and genes implicated with disease severity based on COVID-19 GWAS. Our foundational dataset elucidates the biological impact of severe SARS-CoV-2 infection across the body, a key step towards new treatments.

|
Scooped by
Gilbert C FAURE
April 26, 2021 5:03 AM
|
Tiziana is all set to commence a Phase II trial of its nasal Foralumab in moderate to severe hospitalised Covid-19 patients in Brazil.
As COVID-19 continues to strain public health systems and vaccination programmes race
against new variants that might be more transmissible or capable of evading immune
responses, the urgent need for simple, accessible, and frequent testing remains. Inexpensive,
scalable, and sustainable strategies that allow easily repeatable testing over time
need to be made widely available. This is possible by testing saliva.
Via HAS-veille

|
Suggested by
LIGHTING
April 6, 2021 11:52 AM
|

|
Scooped by
Gilbert C FAURE
March 29, 2021 2:25 PM
|
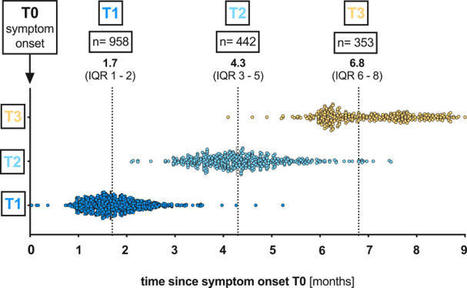
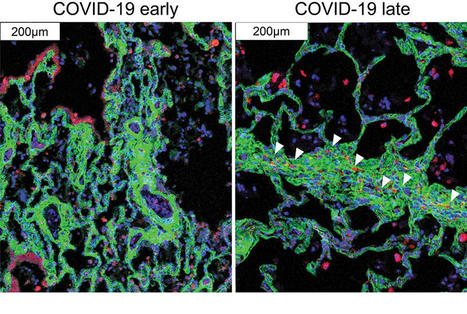
Recent studies have provided insights into the pathology and immune response to coronavirus disease 2019 (COVID-19)1–8. However, thorough interrogation of the interplay between infected cells and the immune system at sites of infection is lacking. We use high parameter imaging mass cytometry9 targeting the expression of 36 proteins, to investigate at single cell resolution, the cellular composition and spatial architecture of human acute lung injury including SARS-CoV-2. This spatially resolved, single-cell data unravels the disordered structure of the infected and injured lung alongside the distribution of extensive immune infiltration. Neutrophil and macrophage infiltration are hallmarks of bacterial pneumonia and COVID-19, respectively. We provide evidence that SARS-CoV-2 infects predominantly alveolar epithelial cells and induces a localized hyper-inflammatory cell state associated with lung damage. By leveraging the temporal range of COVID-19 severe fatal disease in relation to the time of symptom onset, we observe increased macrophage extravasation, mesenchymal cells, and fibroblasts abundance concomitant with increased proximity between these cell types as the disease progresses, possibly as an attempt to repair the damaged lung tissue. This spatially resolved single-cell data allowed us to develop a biologically interpretable landscape of lung pathology from a structural, immunological and clinical standpoint. This spatial single-cell landscape enabled the pathophysiological characterization of the human lung from its macroscopic presentation to the single-cell, providing an important basis for the understanding of COVID-19, and lung pathology in general.

|
Scooped by
Gilbert C FAURE
March 27, 2021 4:16 AM
|
Jerusalem-based scientist expects approval for humans within 6 months, says advance could simplify vaccination drives and even allow home vaccination using pills sent by mail...

|
Scooped by
Gilbert C FAURE
March 27, 2021 4:10 AM
|
Abstract Dysgeusia is the first recognized oral symptom of novel coronavirus disease (COVID‐19). In this review article, we described oral lesions of COVID‐19 patients. We searched PubMed library a...

|
Scooped by
Gilbert C FAURE
March 23, 2021 2:44 PM
|
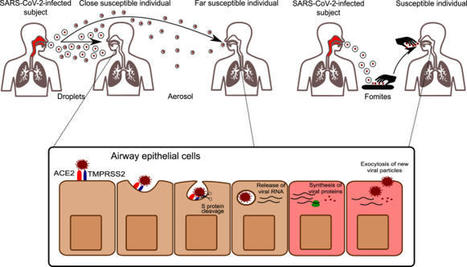
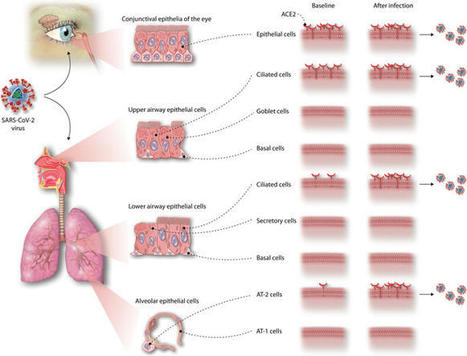
The novel coronavirus SARS-CoV-2 enters into the human body mainly through the ACE2 + TMPRSS2+ nasal epithelial cells. The initial host response to this pathogen occurs in a peculiar immune microenvironment that, starting from the Nasopharynx-Associated Lymphoid Tissue (NALT) system, is the product of a long evolutionary process that is aimed to first recognize exogenous airborne agents. In the present work, we want to critically review the latest molecular and cellular findings on the mucosal response to SARS-CoV-2 in the nasal cavity and in NALT, and to analyze its impact in the subsequent course of COVID-19. Finally, we want to explore the possibility that the regulation of the systemic inflammatory network against the virus can be modulated starting from the initial phases of the nasal and nasopharyngeal response and this may have several clinical and epidemiological implications starting from a mucosal vaccine development.

|
Scooped by
Gilbert C FAURE
March 16, 2021 8:57 AM
|
Rokote Laboratories are seeking investment for the vaccine and are already in discussions about clinical trials although no date has been set.
|

|
Scooped by
Gilbert C FAURE
May 29, 2021 4:38 AM
|
Conventional smoking is known to both increase susceptibility to infection and drive inflammation within the lungs. Recently, smokers have been found to be at higher risk of developing severe forms of coronavirus disease 2019 (COVID-19).

|
Scooped by
Gilbert C FAURE
May 19, 2021 1:50 PM
|
The on-going presence of either shortness of breath, anosmia, ageusia or fatigue as
long-lasting symptoms even in non-hospitalised patients was observed at four and seven
months post-infection and summarised as post-COVID syndrome (PCS).

|
Scooped by
Gilbert C FAURE
May 15, 2021 12:29 PM
|
COVID-19 is more severe in pregnant women and can lead to adverse fetal outcomes.
Through histological and gene expression studies of placentas from infected women,
Lu-Culligan et al. find that maternal SARS-CoV-2 infection during term pregnancy and
delivery is associated with immune activation at the maternal-fetal interface even
in the absence of detectable virus in the placenta.

|
Scooped by
Gilbert C FAURE
May 1, 2021 10:35 AM
|
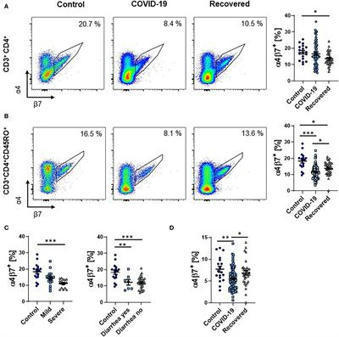
BackgroundInfection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a wide range of symptoms including gastrointestinal manifestations, and intestinal epithelial cells are a target of the virus. However, it is unknown how the intestinal immune system contributes to systemic immune responses in coronavirus disease 2019 (COVID-19).MethodsWe characterized peripheral blood lymphocytes from patients with active COVID-19 and convalescent patients as well as healthy controls by flow cytometry.ResultsThe frequency and absolute number of circulating memory T and B cells expressing the gut homing integrin α4β7 integrin was reduced during COVID-19, whether gastrointestinal symptoms were present or not. While total IgA-expressing B cells were increased, gut-imprinted B cells with IgA expression were stable.ConclusionCOVID-19 is associated with a decrease in circulating adaptive immune cells expressing the key gut homing marker α4β7 suggesting that these cells are preferentially recruited to extra-intestinal tissues independently of α4β7 or that the systemic immune response against SARS-CoV-2 is at least numerically dominated by extraintestinal, particularly pulmonary, immune cell priming.

|
Scooped by
Gilbert C FAURE
April 27, 2021 6:55 AM
|
European researchers used 3D tissue models, or organoids, of the human gut to determine that a particular subpopulation of cells is most affected by SARS-CoV-2. The enhanced understanding of those cells mount an immune response to the virus could enhance the search for new COVID-19 therapies, they...

|
Scooped by
Gilbert C FAURE
April 25, 2021 4:43 AM
|
Can we inhibit the initial stages of the infection of the respiratory tract?

|
Scooped by
Gilbert C FAURE
April 9, 2021 11:14 AM
|
New research into COVID transmission and disease development, as well as a potential game-changing test for sports-related concussions, made saliva this week's top trending clinical topic.

|
Scooped by
Gilbert C FAURE
April 1, 2021 8:30 AM
|
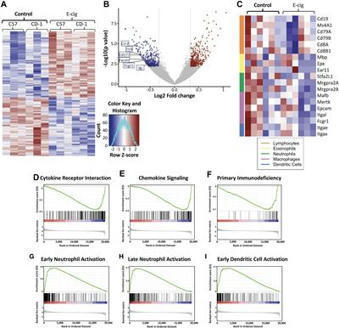
A new imaging technology called ‘high parameter imaging mass cytometry’ developed through a collaborative effort reveals hitherto unseen details in lung pathology due to several infectious diseases including COVID-19.

|
Rescooped by
Gilbert C FAURE
from Virus World
March 28, 2021 3:47 AM
|
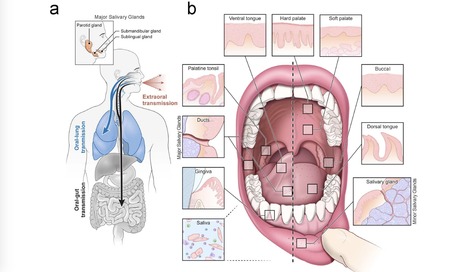
Despite signs of infection—including taste loss, dry mouth and mucosal lesions such as ulcerations, enanthema and macules—the involvement of the oral cavity in coronavirus disease 2019 (COVID-19) is poorly understood. To address this, we generated and analyzed two single-cell RNA sequencing datasets of the human minor salivary glands and gingiva (9 samples, 13,824 cells), identifying 50 cell clusters. Using integrated cell normalization and annotation, we classified 34 unique cell subpopulations between glands and gingiva. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral entry factors such as ACE2 and TMPRSS members were broadly enriched in epithelial cells of the glands and oral mucosae. Using orthogonal RNA and protein expression assessments, we confirmed SARS-CoV-2 infection in the glands and mucosae. Saliva from SARS-CoV-2-infected individuals harbored epithelial cells exhibiting ACE2 and TMPRSS expression and sustained SARS-CoV-2 infection. Acellular and cellular salivary fractions from asymptomatic individuals were found to transmit SARS-CoV-2 ex vivo. Matched nasopharyngeal and saliva samples displayed distinct viral shedding dynamics, and salivary viral burden correlated with COVID-19 symptoms, including taste loss. Upon recovery, this asymptomatic cohort exhibited sustained salivary IgG antibodies against SARS-CoV-2. Collectively, these data show that the oral cavity is an important site for SARS-CoV-2 infection and implicate saliva as a potential route of SARS-CoV-2 transmission. Published in Nature Medicine (March 25, 2021): https://doi.org/10.1038/s41591-021-01296-8
Via Juan Lama

|
Scooped by
Gilbert C FAURE
March 27, 2021 4:14 AM
|
medRxiv - The Preprint Server for Health Sciences...

|
Scooped by
Gilbert C FAURE
March 23, 2021 3:13 PM
|
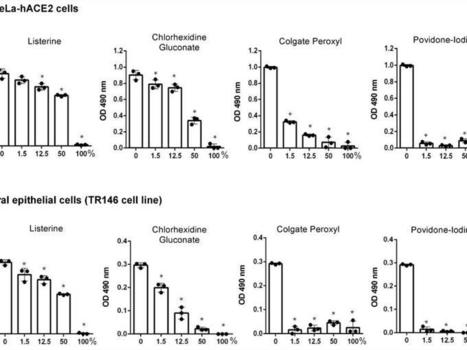
Researchers at Rutgers School of Dental Medicine have found evidence that two types of mouthwash disrupt the COVID-19 virus under laboratory conditions, preventing it from replicating in a human cell.

|
Scooped by
Gilbert C FAURE
March 16, 2021 8:59 AM
|
Altimmune has announced additional preclinical data in regards to its single-dose intranasal COVID-19 vaccine candidate, AdCOVID.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...