 Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
January 4, 10:56 AM
|
Researchers at the Federal University of São Paulo (Unifesp - Universidade Federal de São Paulo) in Brazil have uncovered a previously unrecognized immune-evasion mechanism used by #SARSCoV2, revealing how the #virus directly manipulates host cell #RNA to suppress #antiviral defenses.
▪️ Published in NAR Molecular Medicine, the study shows that beyond conventional immune evasion, SARS-CoV-2 interacts with host RNA in infected #lung cells in a uniquely sophisticated way, disrupting #interferon signaling, a cornerstone of innate #immunity.
▪️ Led by Prof. Marcelo R. S. Briones, the research demonstrates that viral RNA rapidly engages long non-coding RNAs (lncRNAs) - including UCA1, GAS5, and NORAD - immediately after cell entry. Through N⁶-methyladenosine (m⁶A) methylation, the virus alters RNA structure, destabilizing classical base pairing and promoting alternative interactions that weaken RNA-RNA regulation and blunt interferon responses.
▪️ Notably, UCA1 emerged as a central regulatory node, showing altered expression and increased methylation while directly interacting with both the viral #genome and interferon pathway components.
▪️ The work, with key contributions from Cristina Mendes Peter and Caio De Oliveira Cyrino, leveraged Oxford Nanopore Technologies sequencing and #machinelearning analyses to map RNA interactions and methylation patterns in real time, with mathematical support from Fernando Antoneli and Nilmar Moretti.
💡 While fundamentally mechanistic, these findings reshape our understanding of RNA virus #biology and point toward potential therapeutic strategies, including targeting RNA methylation enzymes to restore antiviral immunity.
🗃️ See comments section for reference. | 16 comments on LinkedIn

|
Scooped by
Gilbert C FAURE
February 5, 2025 4:42 AM
|
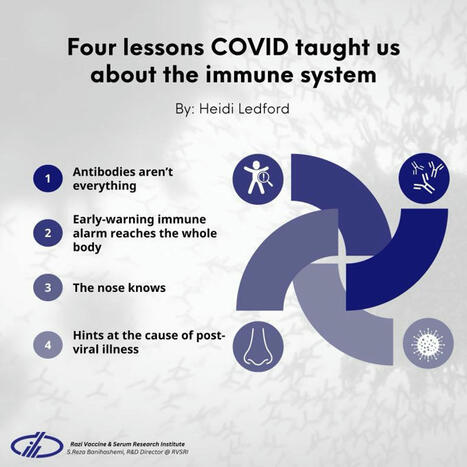
𝐅𝐨𝐮𝐫 𝐥𝐞𝐬𝐬𝐨𝐧𝐬 𝐂𝐎𝐕𝐈𝐃 𝐭𝐚𝐮𝐠𝐡𝐭 𝐮𝐬 𝐚𝐛𝐨𝐮𝐭 𝐭𝐡𝐞 𝐢𝐦𝐦𝐮𝐧𝐞 𝐬𝐲𝐬𝐭𝐞𝐦, 𝐧𝐚𝐭𝐮𝐫𝐞 𝐍𝐄𝐖𝐒📋
✅The COVID-19 pandemic has revealed…

|
Scooped by
Gilbert C FAURE
September 6, 2024 10:56 AM
|
Researchers at the Institut Pasteur in France have developed artificial “lymphoid organ-chips” that recreate much of the human immune system’s response to booster vaccines. The technology, described in an article to be published September 6 in the Journal of Experimental Medicine (JEM), could potentially be used to evaluate the likely effectiveness of new protein and mRNA-based booster vaccines for COVID-19 and other infectious diseases.

|
Scooped by
Gilbert C FAURE
April 18, 2024 5:26 AM
|

|
Scooped by
Gilbert C FAURE
January 24, 2024 6:32 AM
|
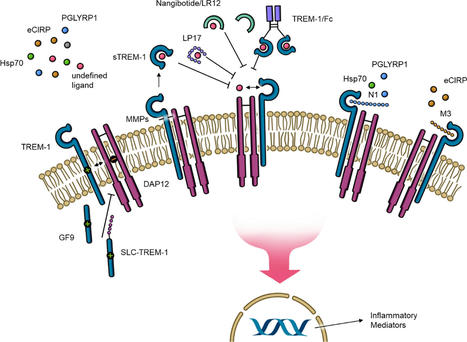
Triggering receptor expressed on myeloid cells-1 (TREM-1) is a pattern recognition receptor and plays a critical role in the immune response. TREM-1 activation leads to the production and release of proinflammatory cytokines, chemokines, as well as its own expression and circulating levels of the cleaved soluble extracellular portion of TREM-1 (sTREM-1). Because patients with sepsis and septic shock show elevated sTREM-1 levels, TREM-1 has attracted attention as an important contributor to the inadequate immune response in this often-deadly condition. Since 2001, when the first blockade of TREM-1 in sepsis was performed, many potential TREM-1 inhibitors have been established in animal models. However, only one of them, nangibotide, has entered clinical trials, which have yielded promising data for future treatment of sepsis, septic shock, and other inflammatory disease such as COVID-19. This review discusses the TREM-1 pathway and important ligands, and highlights the development of novel inhibitors as well as their clinical potential for targeted treatment of various inflammatory conditions.

|
Rescooped by
Gilbert C FAURE
from Virus World
November 25, 2023 2:42 AM
|
An ancient conflict between hosts and pathogens has driven the innate and adaptive arms of immunity. Knowledge about this interplay can not only help us identify biological mechanisms but also reveal pathogen vulnerabilities that can be leveraged therapeutically. The humoral response to SARS-CoV-2 infection has been the focus of intense research, and the role of the innate immune system has received significantly less attention. Here, we review current knowledge of the innate immune response to SARS-CoV-2 infection and the various means SARS-CoV-2 employs to evade innate defense systems. We also consider the role of innate immunity in SARS-CoV-2 vaccines and in the phenomenon of long COVID. Published in Cell. Mol. Immunology (Nov. 20, 2023): https://doi.org/10.1038/s41423-023-01104-y
Via Juan Lama

|
Scooped by
Gilbert C FAURE
October 1, 2023 5:18 AM
|
Celles et ceux qui me font l'honneur de me suivre se souviennent peut-être d'un récent post annonçant la création par Yale University du Centre de l'Infection…

|
Scooped by
Gilbert C FAURE
July 20, 2023 2:58 AM
|
Studies have demonstrated that at least 20% of individuals infected with SARS-CoV-2 remain asymptomatic1–4. Although most global efforts have focused on severe illness in COVID-19, examining asymptomatic infection provides a unique opportunity to consider early immunological features that promote rapid viral clearance. Here, postulating that variation in the human leukocyte antigen (HLA) loci may underly processes mediating asymptomatic infection, we enrolled 29,947 individuals, for whom high-resolution HLA genotyping data were available, in a smartphone-based study designed to track COVID-19 symptoms and outcomes. Our discovery cohort (n = 1,428) comprised unvaccinated individuals who reported a positive test result for SARS-CoV-2. We tested for association of five HLA loci with disease course and identified a strong association between HLA-B*15:01 and asymptomatic infection, observed in two independent cohorts. Suggesting that this genetic association is due to pre-existing T cell immunity, we show that T cells from pre-pandemic samples from individuals carrying HLA-B*15:01 were reactive to the immunodominant SARS-CoV-2 S-derived peptide NQKLIANQF. The majority of the reactive T cells displayed a memory phenotype, were highly polyfunctional and were cross-reactive to a peptide derived from seasonal coronaviruses. The crystal structure of HLA-B*15:01–peptide complexes demonstrates that the peptides NQKLIANQF and NQKLIANAF (from OC43-CoV and HKU1-CoV) share a similar ability to be stabilized and presented by HLA-B*15:01. Finally, we show that the structural similarity of the peptides underpins T cell cross-reactivity of high-affinity public T cell receptors, providing the molecular basis for HLA-B*15:01-mediated pre-existing immunity. The human leukocyte antigen allele HLA-B*15:01 is associated with asymptomatic SARS-CoV-2 infection due to pre-existing T cell immunity.

|
Scooped by
Gilbert C FAURE
June 29, 2023 6:18 AM
|
Antibody-dependent enhancement (ADE) has been shown previously for SARS-CoV-1, MERS-CoV, and SARS-CoV-2 infection in vitro. In this study, the first monoclonal antibody (mAb) that causes ADE in a SARS-CoV-2 in vivo model was identified.

|
Rescooped by
Gilbert C FAURE
from Virus World
March 21, 2023 9:53 AM
|
NIH-funded study suggests need to boost CD8+ T cell response after infection. The magnitude and quality of a key immune cell’s response to vaccination with two doses of the Pfizer-BioNTech COVID-19 vaccine were considerably lower in people with prior SARS-CoV-2 infection compared to people without prior infection, a study has found. In addition, the level of this key immune cell that targets the SARS-CoV-2 spike protein was substantially lower in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Importantly, people who recover from SARS-CoV-2 infection and then get vaccinated are more protected than people who are unvaccinated. These findings, which suggest that the virus damages an important immune-cell response, were published today in the journal Immunity. The study was co-funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and led by Mark M. Davis, Ph.D. Dr. Davis is the director of the Stanford Institute for Immunity, Transplantation and Infection and a professor of microbiology and immunology at Stanford University School of Medicine in Palo Alto, California. He is also a Howard Hughes Medical Institute Investigator. Dr. Davis and colleagues designed a very sensitive tool to analyze how immune cells called CD4+ T cells and CD8+ T cells respond to SARS-CoV-2 infection and vaccination. These cells coordinate the immune system’s response to the virus and kill other cells that have been infected, helping prevent COVID-19. The tool was designed to identify T cells that target any of dozens of specific regions on the virus’s spike protein as well as some other viral regions. The Pfizer-BioNTech vaccine uses parts of the SARS-CoV-2 spike protein to elicit an immune response without causing infection. The investigators studied CD4+ and CD8+ T-cell responses in blood samples from three groups of volunteers. One group had never been infected with SARS-CoV-2 and received two doses of the Pfizer-BioNTech COVID-19 vaccine. The second group had previously been infected with SARS-CoV-2 and received two doses of the vaccine. The third group had COVID-19 and was unvaccinated. The researchers found that vaccination of people who had never been infected with SARS-CoV-2 induced robust CD4+ and CD8+ T-cell responses to the virus’ spike protein. In addition, these T cells produced multiple types of cell-signaling molecules called cytokines, which recruit other immune cells—including antibody-producing B cells—to fight pathogens. However, people who had been infected with SARS-CoV-2 prior to vaccination produced spike-specific CD8+ T cells at considerably lower levels—and with less functionality—than vaccinated people who had never been infected. Moreover, the researchers observed substantially lower levels of spike-specific CD8+ T cells in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Taken together, the investigators write, these findings suggest that SARS-CoV-2 infection damages the CD8+ T cell response, an effect akin to that observed in earlier studies showing long-term damage to the immune system after infection with viruses such as hepatitis C or HIV. The new findings highlight the need to develop vaccination strategies to specifically boost antiviral CD8+ T cell responses in people previously infected with SARS-CoV-2, the researchers conclude. Research published (March 15, 2023) in Immunity: https://doi.org/10.1016/j.immuni.2023.03.005
Via Juan Lama

|
Scooped by
Gilbert C FAURE
January 12, 2023 4:32 AM
|
SARS-CoV-2, the virus responsible for the COVID-19 pandemic, has been associated with substantial global morbidity and mortality. Despite a tropism that is largely confined to the airways, COVID-19 is associated with multiorgan dysfunction and long-term cognitive pathologies. A major driver of this biology stems from the combined effects of virus-mediated interference with the host antiviral defences in infected cells and the sensing of pathogen-associated material by bystander cells. Such a dynamic results in delayed induction of type I and III interferons (IFN-I and IFN-III) at the site of infection, but systemic IFN-I and IFN-III priming in distal organs and barrier epithelial surfaces, respectively. In this Review, we examine the relationship between SARS-CoV-2 biology and the cellular response to infection, detailing how antagonism and dysregulation of host innate immune defences contribute to disease severity of COVID-19. In this Review, Minkoff and tenOever examine the relationship between SARS-CoV-2 biology and innate immunity, and they explore how antagonism and dysregulation of host innate immune defences contribute to COVID-19 disease severity.

|
Scooped by
Gilbert C FAURE
December 8, 2022 11:24 AM
|
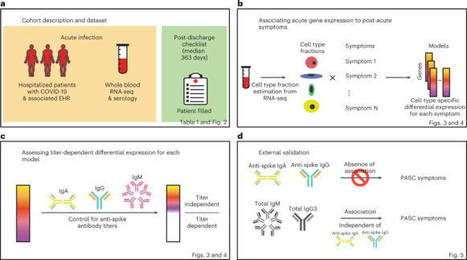
Post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are debilitating, clinically heterogeneous and of unknown molecular etiology. A transcriptome-wide investigation was performed in 165 acutely infected hospitalized individuals who were followed clinically into the post-acute period. Distinct gene expression signatures of post-acute sequelae were already present in whole blood during acute infection, with innate and adaptive immune cells implicated in different symptoms. Two clusters of sequelae exhibited divergent plasma-cell-associated gene expression patterns. In one cluster, sequelae associated with higher expression of immunoglobulin-related genes in an anti-spike antibody titer-dependent manner. In the other, sequelae associated independently of these titers with lower expression of immunoglobulin-related genes, indicating lower non-specific antibody production in individuals with these sequelae. This relationship between lower total immunoglobulins and sequelae was validated in an external cohort. Altogether, multiple etiologies of post-acute sequelae were already detectable during SARS-CoV-2 infection, directly linking these sequelae with the acute host response to the virus and providing early insights into their development. Transcriptomic analyses of acute phase whole blood from a large cohort of patients with COVID-19 identify molecular determinants of post-infection long-term sequelae.

|
Scooped by
Gilbert C FAURE
October 5, 2022 2:42 AM
|
T cells specific for SARS-CoV-2 are thought to protect against infection and development of COVID-19, but direct evidence for this is lacking. Here, we associated whole-blood-based measurement of SARS-CoV-2-specific interferon-γ-positive T cell responses with positive COVID-19 diagnostic (PCR and/or lateral flow) test results up to 6 months post-blood sampling. Amongst 148 participants donating venous blood samples, SARS-CoV-2-specific T cell response magnitude is significantly greater in those who remain protected versus those who become infected (P < 0.0001); relatively low magnitude T cell response results in a 43.2% risk of infection, whereas high magnitude reduces this risk to 5.4%. These findings are recapitulated in a further 299 participants testing a scalable capillary blood-based assay that could facilitate the acquisition of population-scale T cell immunity data (14.9% and 4.4%, respectively). Hence, measurement of SARS-CoV-2-specific T cells can prognosticate infection risk and should be assessed when monitoring individual and population immunity status. The presence of SARS-CoV-2-specific antibodies alone is not an accurate determinant of immunity. In this work, the authors investigate if whole-blood based measurement of SARS-CoV-2 specific T cell responses could prognosticate the risk of possible SARS-CoV-2 infection, and recapitulate their findings in a capillary blood-based assay.
|

|
Scooped by
Gilbert C FAURE
July 29, 2025 1:55 AM
|
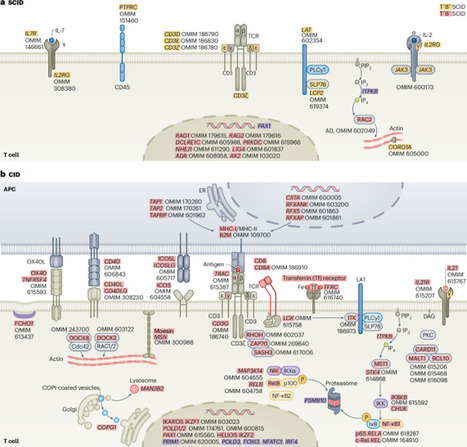
Cui et al. discuss new molecular and cellular insights underlying the pathogenesis of rare human diseases that arise from genetic inborn errors of immunity.

|
Scooped by
Gilbert C FAURE
November 29, 2024 9:56 AM
|

|
Scooped by
Gilbert C FAURE
April 29, 2024 4:26 AM
|
Have you ever wondered why, during the COVID-19 pandemic, the elderly were at greater risk for severe disease, often resulting in hospitalisations, ICU admissions and even death? And why did most children not show any symptoms, despite being infected with the same virus? Then, you’ll find this year’s theme for the International Day of Immunology particularly intriguing: ‘Immunity through the ages’.

|
Scooped by
Gilbert C FAURE
March 1, 2024 5:27 AM
|
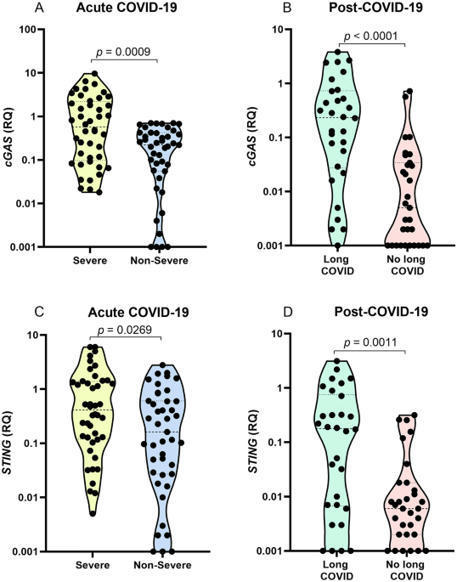
The cGAS-STING pathway appears to contribute to dysregulated inflammation during coronavirus disease 2019 (COVID-19); however, inflammatory factors related to long COVID are still being investigated. In the present study, we evaluated the association of cGAS and STING gene expression levels and plasma IFN-α, TNF-α and IL-6 levels with COVID-19 severity in acute infection and long COVID, based on analysis of blood samples from 148 individuals, 87 with acute COVID-19 and 61 in the post-COVID-19 period. Quantification of gene expression was performed by real-time PCR, and cytokine levels were quantified by ELISA and flow cytometry. In acute COVID-19, cGAS, STING, IFN-α, TNF-α, and IL-6 levels were higher in patients with severe disease than in those with nonsevere manifestations (p < 0.05). Long COVID was associated with elevated cGAS, STING and IFN-α levels (p < 0.05). Activation of the cGAS-STING pathway may contribute to an intense systemic inflammatory state in severe COVID-19 and, after infection resolution, induce an autoinflammatory disease in some tissues, resulting in long COVID.

|
Scooped by
Gilbert C FAURE
December 2, 2023 4:32 AM
|
In addition to the known ACE2 receptor, the SARS-CoV-2 virus can also bind to the RAGE receptor found in white blood cells.
COVID-19, the disease caused by SARS-CoV-2, has caused significant morbidity and mortality worldwide. The betacoronavirus continues to evolve with global health implications as we race to learn more to curb its transmission, evolution, and sequelae. The focus of this review, the second of a three-part series, is on the biological effects of the SARS-CoV-2 virus on post-acute disease in the context of tissue and organ adaptations and damage. We highlight the current knowledge and describe how virological, animal, and clinical studies have shed light on the mechanisms driving the varied clinical diagnoses and observations of COVID-19 patients. Moreover, we describe how investigations into SARS-CoV-2 effects have informed the understanding of viral pathogenesis and provide innovative pathways for future research on the mechanisms of viral diseases.

|
Scooped by
Gilbert C FAURE
August 15, 2023 5:03 AM
|
Long COVID is an often debilitating illness that occurs in at least 10% of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. More than 200 symptoms have been identified with impacts on multiple organ systems. At least 65 million individuals worldwide are estimated to have long COVID, with cases increasing daily. Biomedical research has made substantial progress in identifying various pathophysiological changes and risk factors and in characterizing the illness; further, similarities with other viral-onset illnesses such as myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome have laid the groundwork for research in the field. In this Review, we explore the current literature and highlight key findings, the overlap with other conditions, the variable onset of symptoms, long COVID in children and the impact of vaccinations. Although these key findings are critical to understanding long COVID, current diagnostic and treatment options are insufficient, and clinical trials must be prioritized that address leading hypotheses. Additionally, to strengthen long COVID research, future studies must account for biases and SARS-CoV-2 testing issues, build on viral-onset research, be inclusive of marginalized populations and meaningfully engage patients throughout the research process. Long COVID is an often debilitating illness of severe symptoms that can develop during or following COVID-19. In this Review, Davis, McCorkell, Vogel and Topol explore our knowledge of long COVID and highlight key findings, including potential mechanisms, the overlap with other conditions and potential treatments. They also discuss challenges and recommendations for long COVID research and care.

|
Rescooped by
Gilbert C FAURE
from Virus World
July 1, 2023 1:04 PM
|
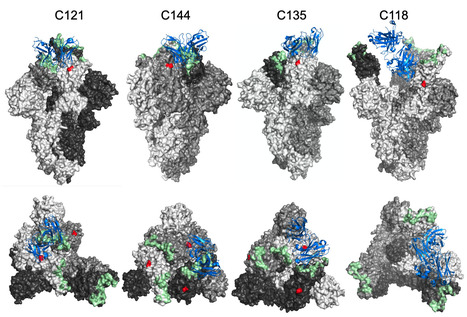
The glycosylation of viral envelope proteins can play important roles in virus biology and immune evasion. The spike (S) glycoprotein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) includes 22 N-linked glycosylation sequons and 17 O-linked glycosites. Here, we investigated the effect of individual glycosylation sites on SARS-CoV-2 S function in pseudotyped virus infection assays and on sensitivity to monoclonal and polyclonal neutralizing antibodies. In most cases, removal of individual glycosylation sites decreased the infectiousness of the pseudotyped virus. For glycosylation mutants in the N-terminal domain (NTD) and the receptor binding domain (RBD), reduction in pseudotype infectivity was predicted by a commensurate reduction in the level of virion-incorporated spike protein. Notably, the presence of a glycan at position N343 within the RBD had diverse effects on neutralization by RBD-specific monoclonal antibodies (mAbs) cloned from convalescent individuals. The N343 glycan reduced overall sensitivity to polyclonal antibodies in plasma from COVID-19 convalescent individuals, suggesting a role for SARS-CoV-2 spike glycosylation in immune evasion. However, vaccination of convalescent individuals produced neutralizing activity that was resilient to the inhibitory effect of the N343 glycan. Preprint at bioRxiv (June 30, 2023): https://doi.org/10.1101/2023.06.30.547241
Via Juan Lama

|
Scooped by
Gilbert C FAURE
May 24, 2023 6:58 AM
|
Interesting study by Belgian researchers reveals risk factors associated with fatal COVID-19 cases among outbreaks elderly patients in nursing homes. Among…

|
Scooped by
Gilbert C FAURE
February 10, 2023 6:18 AM
|
COVID-19 patients have reduced thymic T- cell output, and disease severity negatively
correlates with thymic function. SARS-CoV-2 targeting of thymic epithelial cells induces
the downregulation of critical pathways associated with epithelial cell adhesion and
survival.

|
Scooped by
Gilbert C FAURE
January 7, 2023 12:55 PM
|
In the past 10 years, we have witnessed major advances in clinical immunology. Newborn
screening for severe combined immunodeficiency has become universal in the United
States and screening programs are being extended to severe combined immunodeficiency
and other inborn errors of immunity globally. Early genetic testing is becoming the
norm for many of our patients and allows for informed selection of targeted therapies
including biologics repurposed from other specialties. During the COVID-19 pandemic,
our understanding of essential immune responses expanded and the discovery of immune
gene defects continued.

|
Scooped by
Gilbert C FAURE
December 2, 2022 10:02 AM
|
Host immunity to infection with SARS-CoV-2 is highly variable, dictating diverse clinical outcomes ranging from asymptomatic to severe disease and death. We previously reported reduced type I interferon in severe COVID-19 patients preceded clinical worsening. Further studies identified genetic mutations in loci of the TLR3- or TLR7-dependent interferon-I pathways, or neutralizing interferon-I autoantibodies as risk factors for development of COVID-19 pneumonia. Here we show in patient cohorts with different severities of COVID-19, that baseline plasma interferon α measures differ according to the immunoassay used, timing of sampling, the interferon α subtype measured, and the presence of autoantibodies. We also show a consistently reduced induction of interferon-I proteins in hospitalized COVID-19 patients upon immune stimulation, that is not associated with detectable neutralizing autoantibodies against interferon α or interferon ω. Intracellular proteomic analysis shows increased monocyte numbers in hospitalized COVID-19 patients but impaired interferon-I response after stimulation. We confirm this by ex vivo whole blood stimulation with interferon-I which induces transcriptomic responses associated with inflammation in hospitalized COVID-19 patients, that is not seen in controls or non-hospitalized moderate cases. These results may explain the dichotomy of the poor clinical response to interferon-I based treatments in late stage COVID-19, despite the importance of interferon-I in early acute infection and may guide alternative therapeutic strategies. The interferon response has been shown to be linked to severity of SARS-CoV-2 infection and is an essential component of the immune response to COVID-19. Here the authors stratify patients according to COVID-19 severity and asses the interferon response showing defective responses in severe infection and highlight the importance of assay variables and confounding factors that impact the detection of interferon.
|



 Your new post is loading...
Your new post is loading...






















https://www.scoop.it/topic/immunology?q=covid-19