Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
October 4, 2022 3:06 AM
|
There are several reasons to consider the role of endothelial cells in COVID-19 and other emerging viral infections. First, severe cases of COVID-19 show a common breakdown of central vascular functions. Second, SARS-CoV-2 replicates in endothelial cells. Third, prior deterioration of vascular function exacerbates disease, as the most common comorbidities of COVID-19 (obesity, hypertension, and diabetes) are all associated with endothelial dysfunction. Importantly, SARS-CoV-2's ability to infect endothelium is shared by many emerging viruses, including henipaviruses, hantavirus, and highly pathogenic avian influenza virus, all specifically targeting endothelial cells. The ability to infect endothelium appears to support generalised dissemination of infection and facilitate the access to certain tissues. The disturbed vascular function observed in severe COVID-19 is also a prominent feature of many other life-threatening viral diseases, underscoring the need to understand how viruses modulate endothelial function. We here review the role of vascular endothelial cells in emerging viral infections, starting with a summary of endothelial cells as key mediators and regulators of vascular and immune responses in health and infection. Next, we discuss endotheliotropism as a possible virulence factor and detail features that regulate viruses' ability to attach to and enter endothelial cells. We move on to review how endothelial cells detect invading viruses and respond t

|
Scooped by
Gilbert C FAURE
September 5, 2022 2:37 PM
|
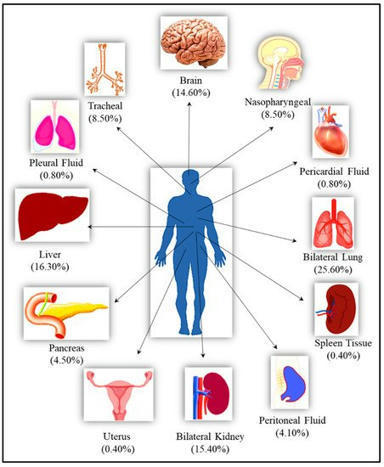
The clinical manifestation of COVID-19 mainly targets the lung as a primary affected organ, which is also a critical site of immune cells activation by SARS-CoV-2. However, recent reports also suggest the involvement of extrapulmonary tissues in COVID-19 pathology.

|
Scooped by
Gilbert C FAURE
August 19, 2022 3:28 AM
|
We have created this teaching resource hub to provide immunology educators with links, videos, worksheets, webinars and other resources that can help explain, describe, supplement and assess immunology content for students from A-level studies up to Master's level. The BSI recognises the particular needs and support that immunology educators require due to the COVID-19

|
Scooped by
Gilbert C FAURE
July 4, 2022 6:34 AM
|
Precise reasons for severe manifestation of SARS-CoV-2 remain unanswered, and efforts have been focused on respiratory system management. Demonstration of unequivocal presence of SARS-CoV-2 in vital body organs by cadaver autopsy was the only way to prove multi-organ involvement.

|
Scooped by
Gilbert C FAURE
June 17, 2022 8:14 AM
|
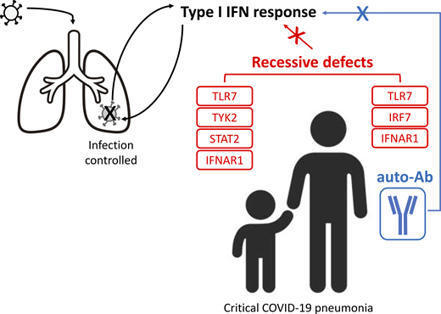
In an international cohort of 112 children hospitalized for moderate to critical COVID-19 pneumonia, we identified 12 children with one of four known recessive

|
Scooped by
Gilbert C FAURE
May 19, 2022 3:38 AM
|
No AccessNewsRequest AccessFull TextA Role for the Vascular Endothelium in Post–Acute COVID-19? Laura P.M.H. de Rooij , Lisa M. Becker and Peter Carmeliet Originally published16 May 2022https://doi.org/10.1161/CIRCULATIONAHA.122.059231Circulation. 2022;145:1503–1505FootnotesThe opinions expressed...

|
Scooped by
Gilbert C FAURE
April 25, 2022 5:21 AM
|
COVID-19 deteriorates type II pneumocytes and damages the alveolar immunologic balancing process through the inadvertent activation of a sequence of localized and general inflammatory responses.Due to an aggregation of uncleaved angiotensin II, the stimulated inflammatory cells cause cytokines synt...

|
Scooped by
Gilbert C FAURE
March 19, 2022 5:25 AM
|
There is a lack of understanding as to why some people suffer from long-lasting symptoms after COVID-19 infection. A new study from Karolinska Institutet in Sweden, the Helmholtz Center Munich (HMGU) and the Technical University of Munich (TUM), both in Germany, now demonstrates that a certain type of immune cell called macrophages show altered inflammatory and metabolic expression several months after mild COVID-19. The findings are published in the journal Mucosal Immunology.

|
Scooped by
Marcelo de Carvalho Bittencourt
February 12, 2022 8:31 AM
|
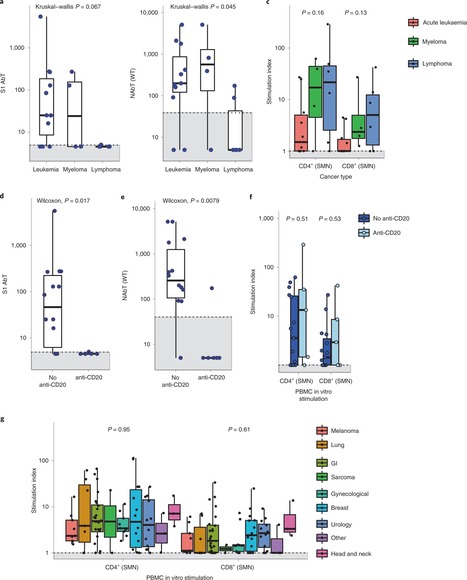
Patients with cancer have higher COVID-19 morbidity and mortality. Here we present the prospective CAPTURE study, integrating longitudinal immune profiling with clinical annotation. Of 357 patients with cancer, 118 were SARS-CoV-2 positive, 94 were symptomatic and 2 died of COVID-19. In this cohort, 83% patients had S1-reactive antibodies and 82% had neutralizing antibodies against wild type SARS-CoV-2, whereas neutralizing antibody titers against the Alpha, Beta and Delta variants were substantially reduced. S1-reactive antibody levels decreased in 13% of patients, whereas neutralizing antibody titers remained stable for up to 329 days. Patients also had detectable SARS-CoV-2-specific T cells and CD4+ responses correlating with S1-reactive antibody levels, although patients with hematological malignancies had impaired immune responses that were disease and treatment specific, but presented compensatory cellular responses, further supported by clinical recovery in all but one patient. Overall, these findings advance the understanding of the nature and duration of the immune response to SARS-CoV-2 in patients with cancer. Turajlic and colleagues assess longitudinal antibody and cellular immune responses against SARS-CoV-2 variants of concern in patients with cancer, following either recovery from SARS-CoV-2 infection or vaccination, in two back-to-back reports from the CAPTURE study.

|
Scooped by
Gilbert C FAURE
January 18, 2022 6:44 AM
|
Hyperactivation of the complement and coagulation systems is recognized as part of the clinical syndrome of COVID-19. Here we review systemic complement activation and local complement activation in response to the causative virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and their currently known relationships to hyperinflammation and thrombosis. We also provide an update on early clinical findings and emerging clinical trial evidence that suggest potential therapeutic benefit of complement inhibition in severe COVID-19. Hyperactivation of the complement system has been implicated in the pathology of COVID-19. Here the authors bring together the latest information on the role of complement in COVID-19 and progress in targeting complement components for treatment of severe disease.

|
Rescooped by
Gilbert C FAURE
from Veille Coronavirus - Covid-19
January 14, 2022 2:58 AM
|
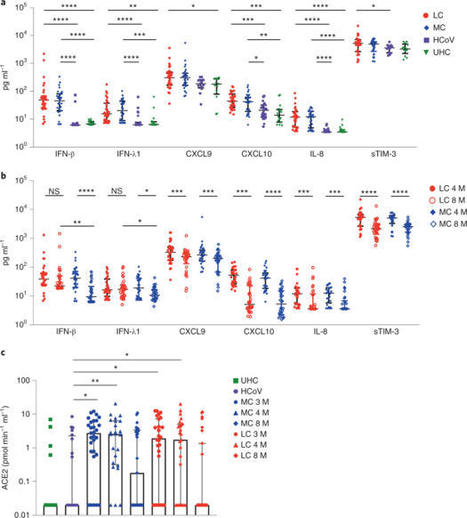
A proportion of patients surviving acute coronavirus disease 2019 (COVID-19) infection develop post-acute COVID syndrome (long COVID (LC)) lasting longer than 12 weeks. Here, we studied individuals with LC compared to age- and gender-matched recovered individuals without LC, unexposed donors and individuals infected with other coronaviruses. Patients with LC had highly activated innate immune cells, lacked naive T and B cells and showed elevated expression of type I IFN (IFN-β) and type III IFN (IFN-λ1) that remained persistently high at 8 months after infection. Using a log-linear classification model, we defined an optimal set of analytes that had the strongest association with LC among the 28 analytes measured. Combinations of the inflammatory mediators IFN-β, PTX3, IFN-γ, IFN-λ2/3 and IL-6 associated with LC with 78.5–81.6% accuracy. This work defines immunological parameters associated with LC and suggests future opportunities for prevention and treatment. Phetsouphanh and colleagues show that individuals with long COVID have persistent activation of the innate and adaptive immune system at 8 months after infection and define a set of analytes associated with long COVID.
Via HAS-veille

|
Scooped by
Gilbert C FAURE
December 29, 2021 3:59 AM
|
Generation of the C3a complement protein fragment by SARS-CoV-2 infection drives differentiation
of a CD16-expressing T cell population that associates with severe COVID-19 disease
outcomes.

|
Scooped by
Gilbert C FAURE
December 8, 2021 6:42 AM
|
Immunological memory is a hallmark of adaptive immunity and facilitates an accelerated and enhanced immune response upon re-infection with the same pathogen1,2. Since the outbreak of the ongoing coronavirus disease 19 (COVID-19) pandemic, a key question has focused on which severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T cells stimulated during acute infection give rise to long-lived memory T cells3. Using spectral flow cytometry combined with cellular indexing of transcriptomes and T cell receptor (TCR) sequencing we longitudinally characterize individual SARS-CoV-2-specific CD8+ T cells of COVID-19 patients from acute infection to one year into recovery and find a distinct signature identifying long-lived memory CD8+ T cells. SARS-CoV-2-specific memory CD8+ T cells persisting one year after acute infection express CD45RA, interleukin-7 receptor α (CD127), and T cell factor-1 (TCF1), but they maintain low CCR7, thus resembling CD45RA+ effector-memory T (TEMRA) cells. Tracking individual clones of SARS-CoV-2-specific CD8+ T cells, we reveal that an interferon signature marks clones giving rise to long-lived cells, whereas prolonged proliferation and mammalian target of rapamycin (mTOR) signaling are associated with clonal disappearance from the blood. Collectively, we describe a transcriptional signature that marks long-lived, circulating human memory CD8+ T cells following an acute virus infection.
|

|
Scooped by
Gilbert C FAURE
September 26, 2022 11:02 AM
|
Demographics of longitudinal study participants. Peripheral blood samples (HLA-A: 02*01+) were obtained from 24 convalescent donors and 9 healthy donors prior to the FDA emergency use authorization of the Johnson & Johnson, Moderna, and Pfizer COVID-19 vaccines.

|
Scooped by
Gilbert C FAURE
August 21, 2022 4:12 AM
|
The emergence of SARS-CoV-2 variants capable of evading neutralizing antibodies have
increased the interest in defining the immunological correlates of disease protection.
Bertoletti, Le Bert, and Tan summarize how SARS-CoV-2-specific T cell magnitude, function
and anatomical localization can affect the their ability to protect against severe
COVID-19.

|
Scooped by
Gilbert C FAURE
August 11, 2022 3:23 AM
|
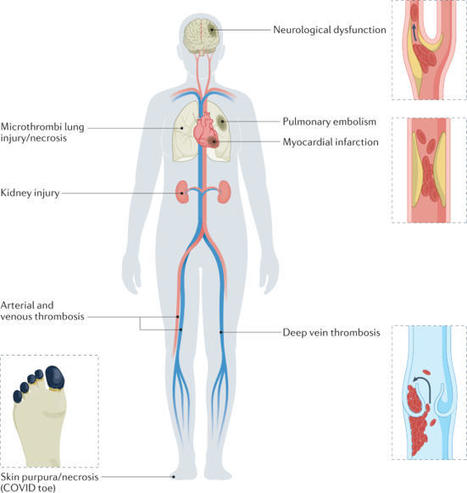
COVID-19-associated coagulopathy (CAC) is a life-threatening complication of SARS-CoV-2 infection. However, the underlying cellular and molecular mechanisms driving this condition are unclear. Evidence supports the concept that CAC involves complex interactions between the innate immune response, the coagulation and fibrinolytic pathways, and the vascular endothelium, resulting in a procoagulant condition. Understanding of the pathogenesis of this condition at the genomic, molecular and cellular levels is needed in order to mitigate thrombosis formation in at-risk patients. In this Perspective, we categorize our current understanding of CAC into three main pathological mechanisms: first, vascular endothelial cell dysfunction; second, a hyper-inflammatory immune response; and last, hypercoagulability. Furthermore, we pose key questions and identify research gaps that need to be addressed to better understand CAC, facilitate improved diagnostics and aid in therapeutic development. Finally, we consider the suitability of different animal models to study CAC. Here, the authors consider our emerging understanding of COVID-19-associated coagulopathy. They focus on the complex interactions between innate immune, coagulation and fibrinolytic pathways that can lead to potentially life-threatening thrombosis following SARS-CoV-2 infection.

|
Scooped by
Gilbert C FAURE
July 1, 2022 2:41 AM
|
Two and a half years into the COVID-19 pandemic, we have gained many insights into the human antibody response to the causative SARS-CoV-2 virus. In this Review, we summarize key observations of humoral immune responses in people with COVID-19, discuss key features of infection- and vaccine-induced neutralizing antibodies, and consider vaccine designs for inducing antibodies that are broadly protective against different variants of the SARS-CoV-2 virus. Qi et al. provide an in-depth analysis of antibody responses generated in response to SARS-CoV-2.

|
Scooped by
Gilbert C FAURE
May 21, 2022 9:52 AM
|
interferon, friend or foe

|
Scooped by
Gilbert C FAURE
May 18, 2022 11:34 AM
|
Author summary Dysregulated immune responses and their associated pathologies are the culprit of severe disease symptoms in response to viral infections. The ability to properly regulate effective and controlled immune responses is a critical feature of preventing severe disease outcomes. Type I interferons are antiviral signaling molecules known to induce potent antiviral immune responses; however, their ability to suppress pathogenic immune responses is poorly understood. Employing a vaginal HSV-2 infection model in mice, we show that type I IFN signaling is critical to preventing the development of severe tissue pathology by suppressing the pathogenic functions of macrophages. In the absence of type I IFNs, these unleashed macrophages produce MMPs that can degrade tissue structure. We show that inhibiting MMPs reduces the severity of immunopathology. We further provide evidence that influenza infection in mice, as well as severe COVID-19 infection in humans, is linked to macrophage and MMP-mediated tissue destruction. Together, our study describes a distinct mechanism through which type I IFNs regulate pathogenic immune responses, and defines MMPs as a potential therapeutic target during severe viral infections. https://medicalxpress.com/news/2022-05-type-i-interferon-immune-rogue-viral.html

|
Scooped by
Gilbert C FAURE
March 27, 2022 4:55 AM
|
The coronavirus disease 2019 (COVID-19) pandemic, causing considerable mortality and morbidity worldwide, has fully engaged the biomedical community in attempts to elucidate the pathophysiology of COVID-19 and develop robust therapeutic strategies.

|
Scooped by
Gilbert C FAURE
March 2, 2022 2:12 PM
|
People suffering from COVID-19 could have several different SARS-CoV-2 variants hidden away from the immune system in different parts of the body, finds new research published in Nature Communications by an international research team. The study's authors say that this may make complete clearance of the virus from the body of an infected person, by their own antibodies, or by therapeutic antibody treatments, much more difficult.

|
Rescooped by
Gilbert C FAURE
from Virus World
January 31, 2022 3:43 AM
|
The entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into human cells is an essential step for virus transmission and development of COVID 19. Although the lung epithelial cells are its initial target, SARS-CoV-2 also can infect endothelial cells. Endothelial cells are the major constituents of the vascular system and cardiovascular complication is a hallmark of severe COVID-19. Angiotensin-converting enzyme 2 (ACE2) is the entry receptor for SARS-CoV-2. However, the possible involvement of other cellular components in the viral entry is not fully understood. A team of researchers from the Boston University School of Medicine (BUSM) has identified extracellular vimentin as an attachment factor that facilitates SARS-CoV-2 entry into human cells. Vimentin is a structural protein that is widely expressed in the cells of mesenchymal origin such as endothelial cells and a potential novel target against SARS-CoV-2, which could block the infection of the SARS-CoV-2. "Severe endothelial injury, vascular thrombosis, and obstruction of alveolar capillaries (tiny air sacs scattered throughout the lungs) are common features of severe COVID-19. Identification of vimentin as a host attachment factor for SARS-CoV-2 can provide new insight into the mechanism of SARS-CoV-2 infection of the vascular system and can lead to the development of novel treatment strategies," said corresponding author Nader Rahimi, Ph.D., associate professor of pathology & laboratory medicine at BUSM. The researchers used liquid chromatography–tandem mass spectrometry (LC-MS/MS) and identified vimentin as a protein that binds to the SARS-CoV-2 spike (S) protein and facilitates SARS-CoV-2 infection. They also found that depletion of vimentin significantly reduces SARS-CoV-2 infection of human endothelial cells. In contrast, over-expression of vimentin with ACE2 significantly increased the infection rate. "More importantly, we saw that the CR3022 antibody inhibited the binding of vimentin with CoV-2-S-protein, and neutralized SARS-CoV-2 entry into human cells," explained Rahimi. Findings Published in P.N.A.S. (Jan. 25, 2022): https://doi.org/10.1073/pnas.2113874119
Via Juan Lama

|
Scooped by
Gilbert C FAURE
January 16, 2022 11:22 AM
|
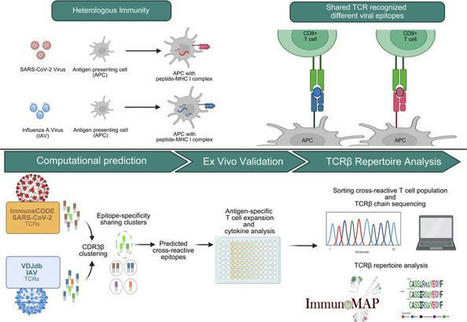
Cross-reactive immune responses to SARS-CoV-2 have been observed in pre-pandemic cohorts and proposed to contribute to host protection. Here we assess 52 COVID-19 household contacts to capture immune responses at the earliest timepoints after SARS-CoV-2 exposure. Using a dual cytokine FLISpot assay on peripheral blood mononuclear cells, we enumerate the frequency of T cells specific for spike, nucleocapsid, membrane, envelope and ORF1 SARS-CoV-2 epitopes that cross-react with human endemic coronaviruses. We observe higher frequencies of cross-reactive (p = 0.0139), and nucleocapsid-specific (p = 0.0355) IL-2-secreting memory T cells in contacts who remained PCR-negative despite exposure (n = 26), when compared with those who convert to PCR-positive (n = 26); no significant difference in the frequency of responses to spike is observed, hinting at a limited protective function of spike-cross-reactive T cells. Our results are thus consistent with pre-existing non-spike cross-reactive memory T cells protecting SARS-CoV-2-naïve contacts from infection, thereby supporting the inclusion of non-spike antigens in second-generation vaccines. While cross-reactive immunity between human coronavirus and SARS-CoV-2 may contribute to host protection, validating evidences are still scarce. Here the authors assess a cohort of 52 donors with immediate-early contact with SARS-CoV-2 to correlate higher frequency of cross-reactive T cells with lower infection rate.

|
Scooped by
Gilbert C FAURE
December 31, 2021 6:47 AM
|
Author summary In this work we describe the prognostic value of early detection of SARS-CoV-2-specific T cell response in acute COVID-19, as patients without a prompt SARS-CoV-2-specific T cell response progress to severe COVID-19. We also show that the presence of specific T cells against SARS-CoV-2 when patients arrive to the emergency room is a protective factor against developing severe COVID-19, independently of the age and gender of the patient, which are two major known contributors to disease outcome. In addition, we show robust cellular and humoral immune responses persist 3 months after real-world vaccination.

|
Scooped by
Gilbert C FAURE
December 23, 2021 10:55 AM
|
It is not fully understood why COVID-19 is typically milder in children1–3. To examine differences in response to SARS-CoV-2 infection in children and adults, we analysed paediatric and adult COVID-19 patients and healthy controls (total n=93) using single-cell multi-omic profiling of matched nasal, tracheal, bronchial and blood samples. In healthy paediatric airways, we observed cells already in an interferon-activated state, that upon SARS-CoV-2 infection was further induced especially in airway immune cells. We postulate that higher paediatric innate interferon-responses restrict viral replication and disease progression. The systemic response in children was characterised by increases in naive lymphocytes and a depletion of natural killer cells, while in adults cytotoxic T cells and interferon-stimulated subpopulations were significantly increased. We provide evidence that dendritic cells initiate interferon signaling in early infection, and identify novel epithelial cell states that associate with COVID-19 and age. Our matching nasal and blood data showed a strong interferon response in the airways with the induction of systemic interferon-stimulated populations, which were massively reduced in paediatric patients. Together, we provide several mechanisms that explain the milder clinical syndrome observed in children.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...