Pharmaceutical companies aren’t currently taking full advantage of the wealth of patient-generated content on social media channels. Deloitte’s Becca Ramble and Katy Balatero outline how these companies can set up successful social listening programmes to uncover patient insights while managing and mitigating risk.
Social networks and online communities play an important role in consumer health management, serving as hubs where patients and caregivers meet to ask questions, share information, and compare experiences with treatments and medications.
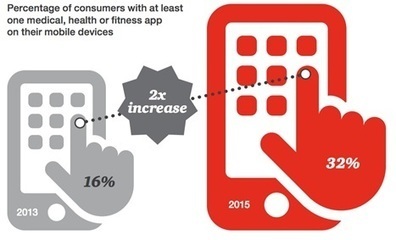
The Deloitte Center for Health Solutions 2015 Survey of US Health Care Consumers found that 52pc of consumers actively search online for health-related information.
Patient-generated content on social networks can highlight the needs, wants and decision considerations of patients and healthcare providers.
This data can be a valuable source of insights for pharmaceutical companies seeking to understand how best to reach, engage, and support patients and healthcare practitioners.
Sharing, but not listening
Companies across a variety of industries are engaging in formal social listening activities.
Social listening involves monitoring social media channels to devise a strategy to help you better influence consumers.
Companies are using insights gleaned from social data to identify market opportunities, inform product and service design, strengthen customer relationships, build engaging customer experiences, manage PR crises, and much more.
Multiple departments, including marketing, customer experience, human resources, corporate communications, sales, and others, are tapping into paid, owned, and earned social media.
These companies recognise that social media listening means tapping into the world’s largest focus group, providing access to current and potential customers and the information they share.
Many pharmaceutical companies have a social media presence. They manage owned social channels, including corporate branded Facebook pages, LinkedIn accounts, and Twitter handles, to share corporate communications, investor relations, event announcements, and press releases. These companies broadcast information and listen on their managed channels, but often their activities end there.
Most are not looking beyond their own channels to understand how, when, and why patients and caregivers are sharing experiences with specific drugs, therapies, diseases, and conditions. Many of these companies are interested in listening and engaging but, when faced with the regulatory risks and considerations, including the discovery and reporting of adverse events, feel that the risks may outweigh the benefits.
While these are real concerns, a thoughtfully-executed social media listening strategy can be used to inform many areas across the business while effectively managing the regulatory risks and considerations.
It’s not all bad
Pharmaceutical companies fear that opening up social listening around their products will expose them to posts where authors share an “undesirable experience associated with the use of a medical product in a patient”, and that they will need to invest heavily in resources to manage adverse event reporting.
In the US, the FDA outlines four criteria that must be present for an adverse event to require reporting:
An identifiable patient: The post contains sufficient information to lead the reviewer to believe that a patient is involved.
An identifiable reporter: The post contains sufficient contact information to allow follow-up by the reviewer, including an email address, telephone number, or mailing address.
A specific medication: The post must mention a specific medication by brand name, or the chemical name of a medication if the compound is unique to one specific pharmaceutical brand.
An adverse event: The post describes a reaction that a “reasonable person” would consider to be an adverse event, such as death, hospitalisation, vomiting, swelling, or any side effect that is either unknown or unexpected with the medication.
However, studies have shown that less than 2pc of all posts mentioning pharmaceutical products and brands contain indicators of potential adverse events.
The structure and nature of most social media posts do not include the level of detail required to meet all four of these criteria. For example, forum threads where participants discuss experiences with a drug but use anonymous usernames or do not provide contact information would not qualify as adverse events. While any pharmaceutical company that engages in brand monitoring should expect to see some adverse event content, Pharmaceutical Commerce wrote that “the volume of adverse events is not likely to exceed what can be handled through existing adverse event reporting channels established for traditional/offline reporting methods”.
The art of social listening
By designing listening and monitoring efforts with their business goals in mind, pharmaceutical companies can develop and deploy strategic listening programmes while managing and mitigating risk. There are several approaches:
Using social media listening and monitoring technologies: Social media listening and monitoring tools allow companies to curate the social media content they need for their specific business goals. By using carefully-curated social media queries, the company can reduce their exposure to unintended content and minimise risk. Many of these tools include functionality to support tagging and reporting activities, if and when an adverse event is discovered, that can streamline the workflow and support the digital paper trail needed for compliance.
Developing visualisations and dashboards: Trend lines, pie charts, and other data visualisations aggregate social media content and organise it by subject matter, such as brand mentions, product groups or types, or patient journey segments. This approach serves up insights and analysis without requiring review of individual posts, thereby removing access and exposure to posts where adverse events may be present.
Exploring unbranded content for insights: Many patients post online about diseases, conditions, or therapy experiences without necessarily mentioning a specific drug or brand. Listening at this level can provide pharmaceutical companies firsthand access to trends, perception shifts, sentiment, and more, while minimising exposure to conversations of brand-related adverse events.
Regardless of the approach, the representatives of the pharmaceutical company who will be accessing and reading individual posts should be trained on adverse event identification. If they are exposed to a post where a potential adverse event is surfaced, it will help them perform due diligence to research the presence of the four FDA criteria.
Reaping the benefits
As brands seek to differentiate themselves through their marketing, products, or communication, a deep understanding of their audiences can play an integral role in meeting those objectives. Social media listening and analysis can inform strategies with actionable information about what people need and want, the language they use, and where they are gathering.
There are many benefits that make social media efforts worthwhile, especially if these programmes are approached with a clear understanding of and management strategy for the risks and regulations involved.
Via
Plus91
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...








![[Exclusif] "Si Bayer veut racheter Monsanto, c'est surtout pour le digital" | Digital Pharma news | Scoop.it](https://img.scoop.it/7V5mp10GQtqBRMsyJ__Gkzl72eJkfbmt4t8yenImKBVvK0kTmF0xjctABnaLJIm9)