Your new post is loading...

|
Scooped by
Juan Lama
June 24, 10:48 AM
|
RetroVirox has launched a Summer Promotion with a 30% discount for antiviral and neutralization services against 4 viruses, including influenza, dengue, human metapneumovirus (HMPV) and respiratory syncytial virus (RSV). Discount applies to services initiated between July 1 and August 31 2025
Contact us at info@retrovirox.com for inquiries and additional info

|
Scooped by
Juan Lama
June 27, 12:41 PM
|
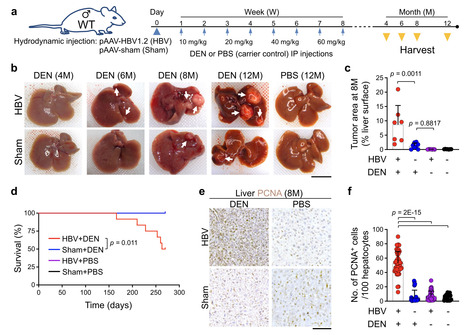
Hepatitis B virus (HBV) infection is associated with hepatitis and hepatocellular carcinoma (HCC). Considering that most HBV-infected individuals remain asymptomatic, the mechanism linking HBV to hepatitis and HCC remains uncertain. Herein, we demonstrate that HBV alone does not cause liver inflammation or cancer. Instead, HBV alters the chronic inflammation induced by chemical carcinogens to promote liver carcinogenesis. Long-term HBV genome expression in mouse liver increases liver inflammation and cancer propensity caused by a carcinogen, diethylnitrosamine (DEN). HBV plus DEN-activated interleukin-33 (IL-33)/regulatory T cell axis is required for liver carcinogenesis. Pitavastatin, an IL-33 inhibitor, suppresses HBV plus DEN-induced liver cancer. IL-33 is markedly elevated in HBV+ hepatitis patients, and pitavastatin use significantly correlates with reduced risk of hepatitis and its associated HCC in patients. Collectively, our findings reveal that environmental carcinogens are the link between HBV and HCC risk, creating a window of opportunity for cancer prevention in HBV carriers. Hepatitis B virus (HBV) infection is associated with hepatitis and hepatocellular carcinoma (HCC). Here, the authors show that HBV promotes HCC formation only in response to environmental carcinogens, involving increased IL-33 signaling and regulatory T cells. Published in NAt. Comm. (June 27, 2025): https://doi.org/10.1038/s41467-025-60894-z

|
Scooped by
Juan Lama
June 26, 11:56 AM
|
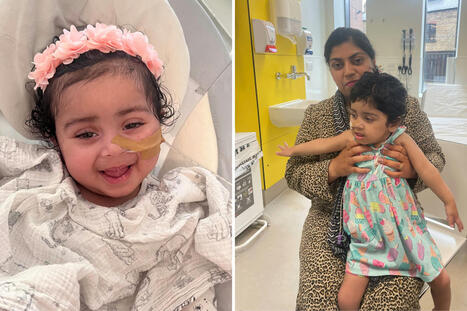
It is the first NHS England commissioned gene therapy in the UK infused directly into the brain. A three-year-old girl has become the youngest patient in the UK to receive a groundbreaking gene therapy for a rare, life-threatening inherited condition. Gunreet Kaur, from Hayes in west London, underwent the pioneering treatment for aromatic l-amino acid decarboxylase (AADC) deficiency, a disorder that severely impacts children’s physical, mental, and behavioural development. Children afflicted with AADC deficiency typically struggle with fundamental bodily controls, including head movement, blood pressure regulation, and heart rate. Gunreet was diagnosed with the condition when she was just nine months old, presenting a significant challenge for her family. However, since receiving the new gene therapy, known as Upstaza, in February 2024, Gunreet has shown remarkable progress. Her mother expressed astonishment at the advancements, which include new movements and vocalisation – milestones she once believed would be unattainable. It is anticipated that Gunreet will continue to make further strides as she grows.The innovative treatment was administered at the world-renowned Great Ormond Street children’s hospital (GOSH) in London. GOSH currently stands as the sole hospital in the UK offering this specific gene therapy to paediatric patients. Sandeep Kaur said Gunreet has made “great progress” since her treatment. “When Gunreet was about seven months old I noticed she wasn’t reaching her milestones at the same age that her older brother did,” she said. “She couldn’t hold her own head up or reach out for items. She cried a lot and always wanted to be held. “Since having the gene therapy, Gunreet has made great progress. “She cries less, smiles more, and can reach for objects. “She can hold her head up and is trying to sit up, she’s recently learned how to roll from her stomach to her back which is fantastic to see. “It means a lot to me that Gunreet was able to have this gene therapy – her general health has improved, she has more co-ordination, she can bring her palms together and is able to move her hand to her mouth.” AADC deficiency is caused by a mutation in the gene that produces the AADC enzyme, this enzyme is needed to produce a neurotransmitter called dopamine which is important in controlling movement. People with AADC deficiency do not have a working version of the enzyme, which means that they have little or no dopamine in the brain. This means that they can suffer developmental delays, weak muscle tone and inability to control the movement of the limbs. It can also lead to painful episodes for affected children. The condition is rare and often deadly, with many children with AADC deficiency not reaching adulthood. The medicine, also known as eladocagene exuparvovec, consists of a virus that contains a working version of the AADC gene. The treatment is delivered by millimetre precision to an exact location in the brain of the patient by a team of medics assisted by a robotic surgery tool. When given to the patient, it is expected that the virus will carry the AADC gene into nerve cells, enabling them to produce the missing enzyme. This is expected to enable the cells to produce the dopamine they need to work properly, which will improve symptoms of the condition. It is the first NHS England commissioned gene therapy in the UK infused directly into the brain. Professor Manju Kurian, consultant paediatric neurologist at GOSH, said “AADC deficiency is a rare condition but often a cruel one that has such a profound impact on children and their carers and families. “We know children with the condition have painful episodes that can last for hours and, as their condition progresses, their life becomes more and more difficult. “It’s incredible to me that I can now prescribe novel gene therapies just as I would prescribe paracetamol and antibiotics. “While the treatment is now available under the NHS at GOSH, we can only do this by working collaboratively across teams inside and outside the hospital, from physios and surgeons to dietitians and speech therapists, alongside partnerships with companies who supply these therapies. “It’s great to see how this treatment has been able to help babies and children across the country like Gunreet. “The natural history of the condition is that most patients cannot fully hold their head or make any developmental progress after that milestone. “Gunreet’s progress over the last year has been really impressive in that context, as well as the virtual disappearance of the eye crises. “We’re hopeful that one day she will be able to talk or walk, as seen in some of the young patients treated in the clinical trial.”

|
Scooped by
Juan Lama
June 23, 11:47 AM
|
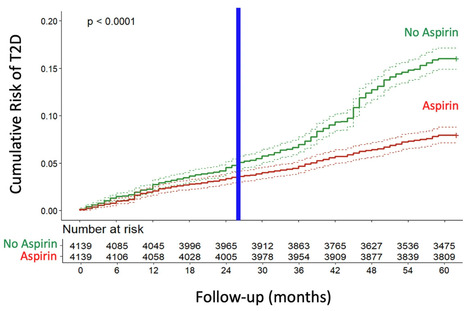
This study aimed to determine whether daily low-dose aspirin reduces the risk of type 2 diabetes (T2D) associated with COVID-19. A longitudinal cohort of 200,000 adults followed from 2018 to 2022 was analyzed, comparing T2D incidence between aspirin users and non-users. Propensity score matching was used to balance the groups. The incidence of T2D was substantially lower in the aspirin group, with Cox regression showing a 52% risk reduction. Kaplan-Meier analysis confirmed a significant divergence in cumulative T2D risk after two years. This protective effect was observed both before and during the COVID-19 pandemic, with a stronger association during the pandemic period. These findings indicate that daily low-dose aspirin significantly reduces the risk of COVID-19-associated new-onset T2D, highlighting the role of inflammation in the pathogenesis of T2D triggered or unmasked by COVID-19. Published (June 18, 2025) NPJ Metabolic Health and Disease: https://doi.org/10.1038/s44324-025-00072-3

|
Scooped by
Juan Lama
June 20, 1:02 PM
|
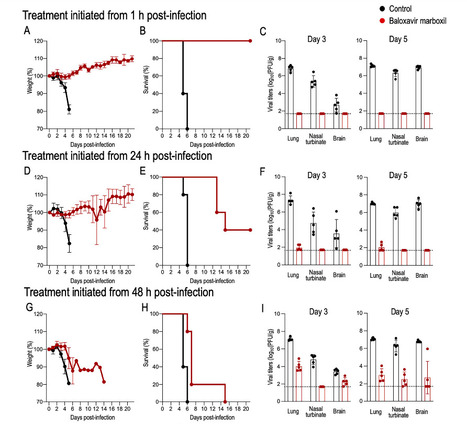
Since the first detection of highly pathogenic avian influenza (HPAI) H5N1 (clade 2.3.4.4b) in U.S. dairy cattle in early 2024, the virus has spread rapidly, posing a major public health concern as the number of human cases continues to rise. Although human-to-human transmission has not been confirmed, experimental data suggest that the bovine H5N1 virus can transmit via respiratory droplets in ferrets, highlighting its pandemic potential. With no vaccines currently available, antiviral drugs remain the only treatment option. Here, we investigate the efficacy of the polymerase inhibitor baloxavir marboxil (BXM) against this virus in mice. We find that early treatment post-infection is effective, but delayed treatment significantly reduces BXM efficacy and increases the risk of BXM resistance, underscoring the importance of timely BXM administration for effective treatment. This study shows that early treatment with baloxavir marboxil is effective against the bovine H5N1 influenza virus, but delayed treatment significantly reduces its effectiveness and increases the risk of emergence of resistant viruses. Published in Nat. Comm (June 20, 2025): https://doi.org/10.1038/s41467-025-60791-5

|
Scooped by
Juan Lama
June 17, 12:51 PM
|
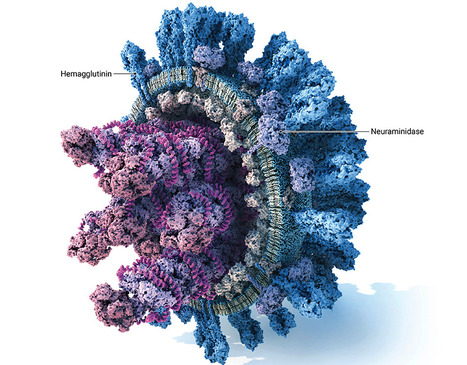
A panzootic of highly pathogenic avian influenza (HPAI) H5N1 viruses from clade 2.3.4.4b has triggered a multistate outbreak in US dairy cattle and an unknown number of human infections. HPAI viruses are handled in specialized biocontainment facilities. Ethical considerations limit certain evolution experiments aimed at assessing viral resistance to potential therapeutics. We have developed a replicating recombinant vesicular stomatitis virus (rVSV) where we replaced its glycoprotein with the hemagglutinin (HA) and neuraminidase (NA) genes of a 2.3.4.4b H5N1 virus (rVSV-H5N1dc2024), which enables these experiments to be performed under standard biosafety considerations. This virus grows to high titers and encodes a fluorescent reporter to track infection. We demonstrate the utility of rVSV-H5N1dc2024 in neutralization experiments, the evaluation of antibody escape, and the characterization of resistance mutations to NA inhibitors. rVSV-H5N1dc2024 or similar viruses may accelerate efforts to develop and evaluate interventions against this emerging threat to human and animal health. IMPORTANCE Highly pathogenic avian influenza H5 viruses have spread globally, established sustained transmission in mammals, and caused human infections. Research on these viruses is restricted to high biocontainment laboratories. We report the characterization and utility of a surrogate, replicating virus that displays the two key influenza virus glycoproteins, hemagglutinin and neuraminidase, and can be safely handled in most research laboratories. This virus is amenable to the evaluation of antiviral antibodies and small-molecule inhibitors and the evolution of viral resistance to these agents. This virus can enable a wider range of researchers to study H5 viruses of pandemic concern.

|
Scooped by
Juan Lama
June 13, 12:32 PM
|
A single infusion of adeno-associated virus (AAV)-mediated gene therapy led to sustained clinical benefit and no late-onset safety concerns in patients with severe hemophilia B, a longitudinal study showed. At a median follow-up of 13 years, factor IX expression remained stable in the 10 men across dose groups, with seven not receiving factor IX prophylaxis, reported Amit Nathwani, MBChB, PhD, of University College London, and colleagues. The median annualized bleeding rate decreased from 14 episodes to 1.5 episodes, representing a median rate reduction by a factor of 9.7, and use of factor IX concentrate decreased by a factor of 12.4, "which considerably alleviated the disease burden," they wrote in the New England Journal of Medicine. "These findings support the long-term safety and efficacy of AAV gene therapy for hemophilia B, thus offering this group of patients a promising and durable treatment option with recently licensed gene-therapy products," Nathwani and team noted. In 2014, Nathwani and his group reported on the successful administration of a single intravenous infusion of a self-complementary, serotype 8 pseudotyped AAV vector encoding a codon-optimized factor IX transgene (scAAV2/8-LP1-hFIXco). Subsequent studies by other investigators confirmed those early results, ultimately leading to the FDA approval of etranacogene dezaparvovec (Hemgenix) and fidanacogene elaparvovec (Beqvez) in adults with severe hemophilia. "However, uncertainties remain regarding the durability of transgene expression," wrote Nathwani and colleagues. "In addition, the long-term safety of AAV-mediated gene transfer remains unclear, and the effect of the humoral immune response to AAV requires ongoing evaluation." The 10 men with severe hemophilia B received a single dose of scAAV2/8-LP1-hFIXco vector, administered through a peripheral vein, in three dose cohorts (low dose: 2×1011 vector genomes [vg]/kg of body weight [n=2], intermediate dose: 6×1011 vg/kg [n=2], or high dose: 2×1012 vg/kg [n=6]) between March 2010 and December 2012. Since 2010, a total of 354 adverse events have been reported; 15 were considered to be associated with AAV gene therapy, including transient elevations in liver aminotransferase levels (of grade 1 or 2) in four of six participants who had been treated with the high vector dose. There were no cases of factor IX inhibition, thrombosis, recurrent transaminitis, or death. Two cases of cancer developed -- lung adenocarcinoma in a patient with a history of smoking 7 years after receipt of gene therapy, and a case of prostate cancer in a 74-year-old patient 11.6 years after gene therapy was administered. Molecular analysis and a review by an expert multidisciplinary team suggested the cancers were not associated with gene therapy. At a median of 3.2 years after gene therapy, all participants had dose-dependent increases in factor IX coagulant activity. At 13 years, factor IX activity remained stable, with mean levels of 1.7 IU/dL in the low-dose cohort, 2.3 IU/dL in the intermediate-dose cohort, and 4.8 IU/dL in the high-dose cohort. Three patients with severe hemophilic arthropathy who had a median of 10 target joints (joints with recurring bleeding episodes) resumed factor IX prophylaxis within 4 years after the receipt of gene therapy due to recurrent spontaneous joint bleeding events. "Factor IX levels of 1 to 3 IU per deciliter in these participants that had been measured at least 3 days after the infusion proved to be insufficient to prevent such bleeding, a finding that highlights the effect of joint health and other biologic factors on outcomes after the receipt of gene therapy," the authors observed... Research Published in N ew England J. Medicine (June 11, 2025): https://www.nejm.org/doi/full/10.1056/NEJMoa2414783

|
Scooped by
Juan Lama
June 12, 11:14 AM
|
Since multiple and unpredicted influenza viruses cause seasonal epidemics and even high-risk pandemics, developing a universal influenza vaccine is essential to provide broad protection against various influenza subtypes. Combined with the mRNA lipid nanoparticle-encapsulated (mRNA-LNP) vaccine platform and chimeric immunogen strategy, we developed a novel cocktail mRNA vaccine encoding chimeric HAs (cH5/1-BV, cH7/3) and intact M2 (termed Fluaxe), which confers broad protection against major circulating IAVs and IBVs, as well as highly pathogenic avian influenza. Two-dose intramuscular immunization of Fluaxe in mice elicited cross-reactive neutralizing antibodies, T cell responses, and long-lived immunity, resulting in robust protection against multiple lethal influenza virus infections and severe acute lung injuries. In particular, intramuscular administration stimulated systemic immunity together with a prominent lung tropism of memory cells. Moreover, Fluaxe immunization inhibited the inflammatory response induced by influenza infection. In summary, we conclude that Fluaxe can elicit broad cross-protection against numerous influenza subtypes. Published in NPJ Vaccines (June 5, 2025): https://doi.org/10.1038/s41541-025-01178-x

|
Scooped by
Juan Lama
June 10, 11:58 AM
|
June 9 (Reuters) - The U.S. Food and Drug Administration on Monday approved Merck's preventive antibody shot to protect infants up to one year of age from respiratory syncytial virus during their first RSV season, the company said. Merck's monoclonal antibody, called clesrovimab and branded as Enflonsia, is the first and only preventive shot that can be administered as a single dose regardless of birth weight in healthy pre-term, full-term and at-risk infants to protect them against mild, moderate and severe RSV. The company told Reuters the therapy will be priced at $556 per dose. RSV is a common respiratory virus that causes seasonal infections such as the flu, but is a leading cause of pneumonia and death in infants and older adults. The approval was based on results from a late-stage trial in which Enflonsia had a comparable safety profile to Swedish Orphan Biovitrum's, Synagis, a monthly injection. Jefferies analyst Akash Tewari said last year the dosing was beneficial since physicians have to forecast an infant's potential weight during RSV season with Sanofi and AstraZeneca's, Beyfortus — the only preventive shot for RSV available in the country for infants and toddlers so far — which makes dose ordering and inventory more complex. The U.S. saw limited supply of antibody Beyfortus up to the 2023 RSV season, but the companies have since tripled production capacity and doubled the number of manufacturing sites. A Sanofi spokesperson said "current supply ... matches the total amount distributed during the entire last season ... We are continuing to manufacture more doses, which will result in a larger overall supply than was available last year." In the U.S., an estimated 58,000–80,000 children younger than five years are hospitalized due to RSV each year, according to the U.S. Centers for Disease Control and Prevention. The CDC currently recommends two immunization options for babies to be protected from severe RSV — an RSV vaccine given to the mother during pregnancy or an RSV antibody given to the baby. Merck expects the drug's shipments to arrive in time for the 2025-2026 RSV season. Merck had earlier on Monday said the CDC's Advisory Committee on Immunization Practices was expected to meet later this month to discuss and make recommendations for the use of Enflonsia in infants. However, Health Secretary Robert F. Kennedy Jr. has fired all members sitting on the CDC panel. Merck was immediately not available for a comment on clarification regarding the meeting. Merck's Press release:

|
Scooped by
Juan Lama
June 9, 11:24 AM
|
Adding IL-12 to mRNA vaccines can lead to longer lasting protective effect and a potential approach for reducing the risk of cancer. Lipid nanoparticle (LNP) mRNA vaccines induce robust immune responses and provide protection against infectious diseases. However, this immunity can be short-lived, resulting in the need for frequent booster vaccinations. In a new study published in Science Immunology titled, “An Il12 mRNA-LNP adjuvant enhances mRNA vaccine–induced CD8 T cell responses,” researchers from the University of Pennsylvania (Penn) found that adding a cytokine produced by various immune cells, known as IL-12, to mRNA vaccines enhanced T-cell response against SARS-CoV-2 and influenza for longer lasting protective effect. The vaccine also improved protection in a mouse melanoma model, offering a potential approach for reducing the risk of cancer. Anthony Phan, PhD, a postdoctoral researcher at Penn and co-lead author, said one practical aspect of an IL-12 mRNA vaccine is being able to reduce the frequency and amount of vaccine. “Anything that can be used to reduce injection frequency and dose-related adverse events is a good thing, and so this allows us to think about that as a strategy,” said Phan. Susan Domchek, MD, director of the Abramson Cancer Center’s Basser Cancer Interception Institute, which provided funding for the study, said cytokine mRNA is “clearly a promising and clever approach to enhance immune stimulation against cancer and pre-cancer lesions.” “We are hopeful this technology can move as fast as possible into the clinic for patients and individuals at risk of cancer,” she continued. IL-12 is naturally produced by the body in response to many infections. Researchers have utilized IL-12 as a vaccine adjuvant to promote immunity against parasitic, viral, and bacterial infections. Designing vaccines that induce strong responses from CD8+ T cells has historically been a challenge, but mRNA vaccines offered a game changer. Data from the last four years show that people who generate strong T-cell responses from the mRNA-based COVID-19 vaccines are less likely to get breakthrough infections or become hospitalized. Christopher Hunter, PhD, distinguished professor of pathobiology at the School of Veterinary Medicine at Penn and corresponding author of the paper, said the study came together through his lab’s interest in cytokine biology and Phan’s particular interest in CD8+ T cells. Additional influencers included the work of Drew Weissman, MD, PhD, 2023 Nobel laureate and director of the Penn Institute for RNA Innovation, on vaccine development, and the expertise of Penn Medicine assistant professor Mohamad-Gabriel Alameh, PhD, on particle design, production, and development of vaccines against bacterial pathogens. “This is why you’re at a university, so you can meet people that you don’t usually interact with, and you find common interests,” said Hunter. Alameh, who met Hunter and Phan on campus, said he quickly realized that combining cytokine-based immune modulation and material engineering is the best route to tailor vaccine responses. Looking ahead, the research team is exploring the impact of other cytokine mRNAs that can be used to modulate the immune response and whether IL-12 could improve vaccine candidates for HIV. In addition, ongoing research with Scott Hensley, PhD, professor of microbiology at Penn Medicine, is focused on the ability to deploy this approach across species for avian influenza. Research published in Science Immunology (June 6, 2025): https://www.science.org/doi/10.1126/sciimmunol.ads1328

|
Scooped by
Juan Lama
June 8, 1:12 PM
|
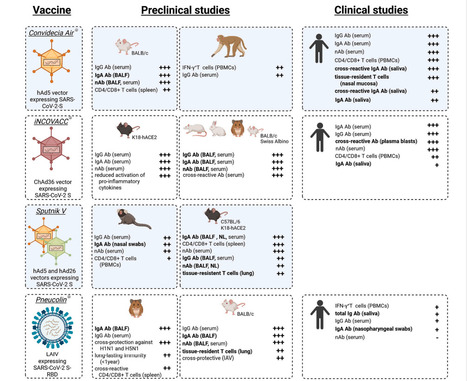
The rapid development and deployment of injectable coronavirus disease 2019 (COVID-19) vaccines - in combination with non-pharmaceutical interventions and development of treatment options - significantly contributed to a decrease in both infection and mortality rates during the pandemic and saved millions of lives. However, injectable vaccines do not robustly and consistently induce a mucosal immune response, which is considered a key factor to prevent infection with and transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Hence, a tremendous effort is being made globally to develop next generation COVID-19 vaccines, which are capable of inducing a robust mucosal immune response in addition to a strong systemic cellular and humoral immune response. Mucosal COVID-19 vaccines have been evaluated successfully in preclinical and clinical trials, in which protection against SARS-CoV-2 infection has been demonstrated. This protective efficacy was associated with the upregulation of secretory IgA antibodies and the maturation of tissue-resident memory cells in the respiratory tract, which, together with an induced systemic immune response, significantly reduced viral replication and transmission in animal models. However, only five active mucosal vaccines (plus one ‘passive’ vaccine) have received approval for human use and robust data on their efficacy in inducing mucosal immune responses in humans and in blocking infection and transmission are missing. This highlights the importance of expanded research in this field. In this review, we aim to summarize what is known about these currently licensed vaccines, with an emphasis on the key findings obtained in both preclinical and clinical studies. Published in Vaccine (August 13, 2025): https://doi.org/10.1016/j.vaccine.2025.127356

|
Scooped by
Juan Lama
June 5, 1:19 PM
|
Live-animal markets are a natural laboratory for viruses to evolve and spark deadly outbreaks, yet scientists lack support to study the risks they pose. In the bustling Jatinegara market in Jakarta, cages are stacked three metres high, holding creatures from all corners of Indonesia and beyond. Bats, raccoon dogs, macaques and songbirds — sold as pets or food — are crammed together, their musky odours mingling with the stench of urine and faeces in the damp tropical air. For decades, public-health experts have warned about the risks of infectious diseases jumping from animals to humans in markets such as Jatinegara, which are part of a global industry worth hundreds of billions of dollars annually. These markets remain “the best way of transmitting diseases”, says James Wood, a veterinary epidemiologist at the University of Cambridge, UK. Jatinegara’s location, in an international travel hub with a population of 11 million people, increases that risk considerably. The world is still recovering from the COVID-19 pandemic, which many researchers say probably started, or was at least amplified, at a market selling live animals in Wuhan, China. Yet the wildlife trade still thrives in many parts of the globe. China banned the farming and trading of most wildlife species for food in 2020, but these practices have simply gone underground. “We are back to business as usual,” says Vincent Nijman, a conservation biologist at Oxford Brookes University, UK, with “millions and millions of animals being traded on a daily basis”. The wildlife trade acts as a vast global network of unregulated natural laboratories, through which potential pathogens freely circulate, evolve and ultimately congregate in urban centres, says Andrew Cunningham, a wildlife epidemiologist at the Institute of Zoology in London. “It’s the scariest thing we are doing,” he says. Before the pandemic, there was a strong emphasis on identifying new viruses in the wild. These efforts were driven by the idea that it might be possible to predict which viruses could spark major disease outbreaks. But many researchers now say that this assumption was flawed. Increasingly, scientists are looking to human–wildlife interfaces — including markets and the trade networks that supply them — as crucial sites for the study of zoonoses, human diseases caused by pathogens that normally infect other species. A handful of research groups are now working to understand how pathogens jump between species, why some jumps cause outbreaks and others don’t, and what kinds of intervention can reduce the risks. But such work is costly, can be dangerous and demands sustained support, which has become increasingly hard to secure. Without investing in such research, “you’re really flying blind”, says Maria Van Kerkhove, head of the emerging diseases and zoonoses unit at the World Health Organization in Geneva, Switzerland. “You’re making recommendations that may not be the most appropriate.”....

|
Scooped by
Juan Lama
June 4, 12:25 PM
|
Tracking measles through wastewater is giving health officials a new window into where the virus is spreading. Measles cases in the U.S. have been rare in recent decades, thanks to a strong childhood vaccination program. But a few cases inevitably pop up each year as travelers bring the virus in from other countries and infect unvaccinated people, primarily children. Those cases are no longer blips. Now that the measles vaccination rate is dropping precipitously across the U.S.—due in part to anti-vaccine sentiments—cases are rising. So far in 2025, 14 outbreaks have been reported in 33 states, according to the U.S. Centers for Disease Control and Prevention (CDC). (By comparison, in 2024, there were just 16 outbreaks reported during the entire year.) Scientists may now have a new way to catch cases. For the first time, researchers have posted national information on where the measles virus is showing up in wastewater. Wastewater surveillance is a useful public-health tool because it provides an objective glimpse into where a given virus is causing infections—often before traditional testing methods. For viruses like measles, which infected people shed in urine, feces, or saliva, it can provide a critical heads-up for health officials. “It gives us a finger-to-the-wind weather map of what is happening with infectious diseases,” says Dr. Marlene Wolfe, assistant professor at Emory and principal investigator and co-program director of WastewaterSCAN, an academic and commercial group that includes researchers from Stanford University, Emory University, and Verily (which is Alphabet Inc.'s research organization). Adding measles to the menu of wastewater tests WastewaterSCAN began testing sewage in the U.S. for the COVID-19 virus in 2020 and has since added other disease-causing microbes including influenza, RSV, human metapneumovirus (HMPV), norovirus, enterovirus, mpox, Candida auris, and hepatitis A. This spring, the scientists began developing a test for picking up signs of the active or "wild type" measles virus that is causing outbreaks in the U.S. The test uses samples from nearly 150 sewage sites across the country and can pick up signs of measles within 48 hours. So far, they have detected it in three sites: one day in Hollywood, Md.; four days toward the end of May in Sacramento; and twice in mid-May in Stamford, Conn. WastewaterSCAN is only one such wastewater surveillance network in the country, though it's the first to test for measles on a national level. The CDC launched its National Wastewater Surveillance System in 2020 and includes more than 1,400 sites that cover 150 million Americans. Some state health departments—including in Texas, where the current measles outbreaks began—also test wastewater samples. WastewaterSCAN collaborates with the CDC, but the CDC does not yet test for measles in wastewater. Why wastewater monitoring may be especially useful for measles Doctors who see patients with measles must report the case to the CDC so the agency can track it and respond to any outbreaks. But it takes time for people to develop symptoms, seek medical care, get tested, and then have their case reported if the test is positive. In addition, the first symptoms of measles are common ones like fever, runny nose, and cough—before the telltale rash appears. Plus, not everyone may get sick enough to know they have measles, so their cases may go unreported. Still others may get sick but not have access to health care and therefore never seek medical help. Wastewater monitoring bypasses those hurdles and can theoretically catch evidence of the virus much earlier. That could be especially helpful with a highly contagious disease like measles, in which one infected person can quickly spread the virus to as many as 18 others. “This is anonymous testing that combines everyone in a community,” says Wolfe. “And for measles, it’s likely that shedding [of the virus] into wastewater is happening before people necessarily recognize what they have.” How wastewater data can help health officials This type of tracking for measles is too new to know yet whether it can detect cases in a region before people start testing positive, Wolfe says. But wastewater surveillance was able to pick up signs of the COVID-19 virus days or sometimes even weeks before cases began appearing in hospitals and testing labs. Over time, as more data are collected on measles, health experts can begin to look for trends and patterns, such as increases in the number of positive samples. That could help public-health departments focus resources like vaccines in areas where infections are spreading. If the number of positive samples start to decline, that information could also give them a heads up when infections are under control. What to do if cases are detected near you Getting vaccinated is the best way to protect yourself against measles infection, and the immunization provides close to lifelong immunity. Making sure that anyone who is eligible is up to date on their measles vaccines can help them avoid infection.
|

|
Scooped by
Juan Lama
Today, 11:31 AM
|
US biomedical agency’s public-access policy kicks in on 1 July. Nature talks to specialists about how to comply. From 1 July, researchers funded by the US National Institutes of Health (NIH) will be required to make their scientific papers available to read for free as soon as they are published in a peer-reviewed journal. That’s according to the agency’s latest public-access policy, aimed at making federally funded research accessible to taxpayers. Established under former US president Joe Biden, the policy was originally set to take effect on 31 December for all US agencies, but the administration of Biden’s successor, Donald Trump, has accelerated its implementation for the NIH, a move that has surprised some scholars. That’s because, although the Trump team has declared itself a defender of taxpayer dollars, it has also targeted programmes and research projects focused on equity and inclusion for elimination. And one of the policy’s main goals is to ensure equitable access to federally funded research. The move means that universities will have less time to advise their researchers on how to comply with the policy, says Peter Suber, director of the Harvard Open Access Project in Cambridge, Massachusetts. There is usually “some confusion or even some non-compliance after a new policy takes effect, but I think universities will eventually get on top of that”, he says. The NIH policy has been welcomed by open-access (OA) advocates, and reflects a global shift towards making publicly funded research freely available. Since 2021, several European science agencies that provide funding for researchers have required grant holders to make their papers OA immediately. What is the new policy? Scientific papers that result from projects funded by the NIH must be deposited in the agency’s digital repository, PubMed Central, and made freely available there immediately after publication in a peer-reviewed journal. Previously, the NIH, the world’s largest public funder of biomedical research, allowed journals to restrict access to papers for an embargo period of up to 12 months before making them publicly available in PubMed Central. The policy applies to manuscripts accepted for publication on or after 1 July, and the version deposited can be either the ‘accepted’ manuscript (including all revisions resulting from the peer-review process) or the final published article. What options do authors have? Scientists can take one of several routes to make their papers immediately free to read. For authors already publishing in an OA journal (or the OA portion of a hybrid journal), the policy brings little change: when researchers’ papers are posted online by the journal, they are made freely available to readers. These authors just need to ensure that their papers are deposited in PubMed Central as soon as they are published. And “if you’re publishing open access, a lot of those publishers have mechanisms in place to do the deposit for you”, says Lisa Hinchliffe, a librarian and academic at the University of Illinois Urbana–Champaign. Authors publishing in closed-access journals (or the closed sections of hybrid journals), which require people to have a subscription to read papers, will be most affected by the change, Hinchliffe says. Researchers will have to make sure that the agreement they sign with the publisher complies with the NIH policy, meaning that they are allowed to deposit the full version of the paper in PubMed Central without embargo. They might also need to submit the paper to PubMed Central themselves, because some publishers have indicated that they will not offer this service. What issues might researchers encounter? Several publishers, including Elsevier and Springer Nature, require that papers published in closed-access journals remain available only to subscribers for an embargo period — 6 or 12 months, for instance — before they can be placed in repositories such as PubMed Central (Nature’s news team is editorially independent of its publisher, Springer Nature). “It’s very likely that not all publisher agreements will be compatible with the new policy, and that creates a difficult situation — both for the publisher and for the author,” Suber says. These publishers might steer authors towards their OA journals (or the OA parts of their hybrid journals) to comply with the policy. However, OA journals make their money by asking for article-processing charges (APCs), which can be anywhere from hundreds to thousands of dollars. Authors switching to publishing in an OA journal would be responsible for paying those APCs. This is what happened when Plan S — an OA initiative launched by a coalition of mostly European funders — went into effect, Hinchliffe says. “Authors should know that in advance, and if they don’t want to pay the APC, but still want to comply with the NIH policy, then they have to go somewhere else,” Suber says. Some authors might not need to worry about APCs, however. In preparation for the NIH policy coming into effect, some universities have been putting in place agreements with publishers that allow their researchers to publish OA papers at no cost to them, generally through contracts where universities shift funds previously spent on subscriptions to supporting OA publishing. The Big Ten Academic Alliance, a consortium of US research universities, announced one such agreement with Springer Nature last month. Not only are we prepared for the transition to OA, says Maurice York, director of Library Initiatives for the Big Ten alliance, but “it’s what we’ve been pushing for”. Furthermore, some funders cover APCs. According to the NIH’s policy, the agency will allow authors to include ‘reasonable costs’ associated with publication, such as APCs, in their project budgets....

|
Scooped by
Juan Lama
June 27, 12:22 PM
|
Thimerosal is found in only a small fraction of US vaccine doses but has long been viewed with suspicion by the anti-vaccination community. Vaccine advisers appointed by US health secretary Robert F. Kennedy Jr have recommended against the use of influenza vaccines containing a mercury-based preservative called thimerosal — fulfilling a long-held goal of the anti-vaccine movement. The vote’s practical effects in the United States are limited, because most US flu vaccine doses are already free of thimerosal, which is only present in multi-dose vials, according to the US Centers for Disease Control and Prevention (CDC). And the ingredient was removed from all childhood vaccines in 2001. But thimerosal has long been a target of the anti-vaccine movement, despite strong evidence supporting its safety in the low doses found in vaccines. Public-health officials and scientists say that the vote’s symbolic effects are powerful as they struggle to maintain public confidence in vaccines. “We are talking about such a small amount of vaccine” containing thimerosal, says Chrissie Juliano, executive director of Big Cities Health Coalition, an association of US health departments, in Takoma Park, Maryland. “What this does is sow mistrust. It confuses the public.” “It was an anti-science recommendation,” says Paul Offit, the director of the Vaccine Education Center at the Children’s Hospital of Philadelphia in Pennsylvania. “We have the information we need to tell us that thimerosal, at the level contained in vaccines, is a trivial contribution to the mercury that we’re exposed to every day.” The US Department of Health and Human Services, which includes the CDC, did not respond to a request for comment by publication time. Handpicked panel The decision came today during the first meeting of the newly chosen Advisory Committee on Immunization Practices (ACIP), a panel that recommends which vaccines US residents should receive and when. Most US health-insurance programmes, which fund health care for US residents, are required to provide vaccines recommended by the ACIP at no cost. Earlier this month, Kennedy, a vaccine sceptic, fired all 17 members of the committee and appointed 8 new ones, some of whom have expressed scepticism about vaccines. (One new member stepped down shortly before the meeting.) Before the vote, the committee heard a presentation by Lyn Redwood, a nurse practitioner and president emeritus of the anti-vaccine group Children's Health Defense in Franklin Lakes, New Jersey, founded by Kennedy. She advocated for the removal of the ingredient from US vaccines, pointing to various studies to make her case. Offit, who is a former member of the ACIP, says that subject-matter experts typically vet presentations before the meeting — a step that was not followed in this case — and probably would have flagged some of the data Redwood presented as inaccurate. A version of Redwood’s slides posted before the meeting contained a reference to a paper that doesn't exist, according to Reuters. The reference was later removed. Some members of the committee echoed Redwood’s claims about the safety of thimerosal, but member Cody Meissner, a paediatrics researcher at the Dartmouth Geisel School of Medicine in Lebanon, New Hampshire, criticized the meeting’s focus on thimerosal. “I’m not quite sure how to respond to this presentation. This is an old issue that has been addressed in the past,” he said. “Of all the issues that ACIP needs to focus on, this is not a big issue,” he added. His was the only dissenting vote on the recommendation against thimerosal-containing vaccines. Meissner expressed concerns that the ACIP’s recommendation could impact access to vaccines in other countries. “The recommendations that the ACIP makes are followed among many countries around the world,” he noted, and removing thimerosal from all vaccines could increase costs. Multi-dose vials, which are the ones that still contain the ingredient, are generally cheaper and easier to store and use in mass vaccination programs.

|
Scooped by
Juan Lama
June 25, 11:21 AM
|
Influenza vaccines could soon get some serious competition—or assistance. A new clinical trial shows a single shot of a long-lasting flu drug may protect people for an entire season, and it might do so more effectively than vaccines. In a study that enrolled 5000 healthy adults before the flu season started and followed them until after it ended, the highest dose of the drug provided 76.1% protection from disease, compared with a placebo shot, its developer, Cidara Therapeutics, reported in a press release and on an investor call yesterday. “This is one of the most exciting recent advances for influenza prevention,” says Kathleen Neuzil, a veteran influenza vaccine researcher recently forced out of her job as head of the Fogarty International Center at the National Institutes of Health by President Donald Trump’s administration. The drug’s promise echoes that of the anti-HIV drug lenacapavir, which protects people from infection for 6 months and last week won approval from the Food and Drug Administration as a so-called pre-exposure prophylactic. Called CD388, the flu shot contains a reformulated version of zanamivir, a flu drug also known as Relenza that GSK brought to market in 1999 and is approved to both treat and prevent disease. Zanamivir, which must be inhaled daily, targets neuraminidase, an enzyme made by the virus that frees newly made virions from the surface of infected cells. To create a longer lasting version, Cidara developed a chemical variant of the drug and attached several copies of it to a piece of an antibody called the Fc fragment, which is engineered to resist being broken down by the human body. The study, which took place in the United States and the United Kingdom, recruited adults who had not received the influenza vaccine. They were given one of three different doses of CD388 or a placebo shot under the skin between September and December 2024. Over the next 24 weeks, the researchers tallied the number of participants who had respiratory symptoms, such as coughing and a fever, and a confirmed test for the influenza virus. All three doses provided what Cidara’s chief medical officer, Nicole Davarpanah, described as “highly statistically significant protection,” although the lowest had only 57.7% efficacy and the middle dose 61.3%. No serious side effects occurred at any dose. Seasonal influenza vaccines, which are given either by injection or as a nasal spray, are designed to protect against at least three of the seasonal strains that circulate each winter. Their effectiveness at preventing disease depends heavily on how well they match the flu strains going around in the human population but average only about 40% because mutations in flu viruses often allow them to dodge immune responses. In contrast, CD388 is effective against a wide variety of strains, a study in mice published by Cidara researchers the 17 March issue of Nature Microbiology shows. The drug attacks a region of neuraminidase that cannot easily change without compromising viral fitness. But Neuzil cautions that although resistance to neuraminidase inhibitors is rare in flu viruses, it does occur, and Cidara’s press release did not address the issue. “This would be important to follow,” she says. Epidemiologist Arnold Monto of the University of Michigan School of Public Health says he anticipated Cidara’s positive results, based on a study he helped conduct more than 25 years ago. Inhaling zanamivir every day during the flu season offered 84% protection, Monto and his co-workers reported in JAMA in 1999. Those findings went nowhere because most people don’t want to take a daily drug to prevent the flu, Monto says. But a single injection may attract a large market. “This strategy is very attractive in many ways,” he says. Monto adds that much will depend on the drug’s future price, which the company did not want to discuss. “Flu vaccines are very cheap,” he notes. And several research groups are working to develop “universal” flu vaccines that work against virtually all strains and pack more punch than existing ones. Other drugs to prevent influenza are also in the works, including one being made by Cerberus Therapeutics that delivers zanamivir via “nanobodies”—tiny antibodies derived from camels that are relatively easy to produce in massive quantities—says immunologist and Cerberus Co-Founder Hidde Ploegh. Although he calls CD388 a “valuable approach” and the new data “compelling,” Ploegh believes Cerberus will have an advantage because its drug is squirted up the nose. “If you think of ease of distribution, compliance, and acceptance, then I think any approach that avoids needles is preferable,” he says. Then again Cerberus has yet to reach human studies. Cidara plans to launch large-scale efficacy trials of CD388 in the Southern Hemisphere’s flu season, which starts in early 2026. Those studies will allow participants to also get vaccinated against flu, if they want; the two interventions combined could offer even better protection. The trial will focus on people with compromised immune systems or chronic conditions that make them particularly vulnerable to severe influenza. “If the inhibitor can be manufactured at a large scale at a reasonable price, it could be very helpful as a prophylaxis for high-risk people,” says flu research Adolfo García-Sastre of the Icahn School of Medicine at Mount Sinai. “It’s a very interesting technology.”

|
Scooped by
Juan Lama
June 20, 1:25 PM
|
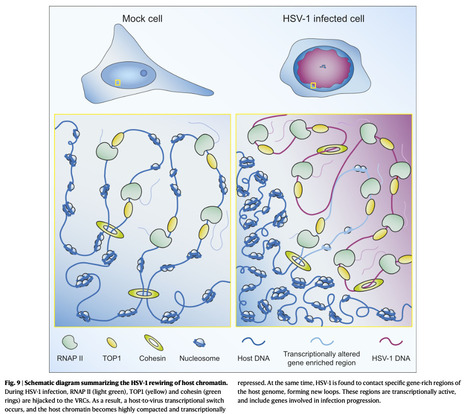
Herpes simplex virus type 1 (HSV-1) remodels the host chromatin structure and induces a host-to-virus transcriptional switch during lytic infection. We combine super-resolution imaging and chromosome-capture technologies to identify the mechanism of remodeling. We show that the host chromatin undergoes massive condensation caused by the hijacking of RNA polymerase II (RNAP II) and topoisomerase I (TOP1). In addition, HSV-1 infection results in the rearrangement of topologically associating domains and loops, although the A/B compartments are maintained in the host. The position of viral genomes and their association with RNAP II and cohesin is determined nanometrically. We reveal specific host–HSV-1 genome interactions and enrichment of upregulated human genes in the most contacting regions. Finally, TOP1 inhibition fully blocks HSV-1 infection, suggesting possible antiviral strategies. This viral mechanism of host chromatin rewiring sheds light on the role of transcription in chromatin architecture. Here, the authors show how HSV-1 rewires the host cell’s chromatin architecture during lytic infection by hijacking transcription machinery and forming contacts between viral and host genomes. Published in Nature Comm. (June 19, 2025): https://doi.org/10.1038/s41467-025-60534-6

|
Scooped by
Juan Lama
June 19, 1:00 PM
|
A drug called lenacapavir, administered in two injections a year, offers protection from HIV comparable to daily pills. One looming question: Will it be affordable for lower resource countries? A drug with the potential to drastically curb the HIV epidemic just cleared its first regulatory hurdle. On Wednesday, the Food and Drug Administration approved lenacapavir for the prevention of HIV. Clinical trial data from last year suggest just two injections a year provide near-complete protection against an HIV infection. "It's a milestone moment in the history of HIV," says Daniel O'Day, chairman and CEO of Gilead Sciences, which manufactures the drug, which was already approved to treat HIV infection. "In our opinion, it's the best tool yet in helping end the HIV epidemic for everyone, everywhere." Others agree. In 2024, Science hailed lenacapavir as its "Breakthrough of the Year" in 2024. The twice-yearly injection offers a more convenient alternative to the current standard of care for HIV prevention, a daily pill called Truvada. This pre-exposure prophylaxis (PrEP) is 99% effective at preventing HIV infection in clinical trials, but some people face significant barriers in taking a daily pill. One study found oral PrEP's was only 26% effective in certain groups, in part because of skipped doses. "Even though the pills work, the Achilles heel of that strategy is that people were not adhering to taking the pills as prescribed," says Onyema Ogbuagu, an infectious disease researcher at Yale University. A twice-a-year treatment could reach substantially more people, especially those who face stigma for taking a daily medication. For instance, researchers found that some single women in South Africa say taking a daily pill raises suspicion among their partners. But the cost of the drug — roughly $28,000 a year — could price out many. While Gilead is taking steps to broaden access, the high price coupled with the U.S.'s steep cuts to foreign aid could prevent people in countries with the highest HIV burden from benefiting...

|
Scooped by
Juan Lama
June 16, 12:30 PM
|
Sarepta halts Elevidys shipments for non-ambulatory DMD patients after a second patient death in three months post gene therapy treatment. Sarepta Therapeutics said it has temporarily suspended shipments of Elevidys® (delandistrogene moxeparvovec-rokl) for infusion in non-ambulatory patients following the second death in three months of a patient with Duchenne muscular dystrophy (DMD) following treatment with the marketed gene therapy. The patient, whose age was not disclosed, was a non-ambulatory individual with DMD. According to Sarepta, the patient was the second to succumb to acute liver failure (ALF) after being treated with Elevidys, which is the only gene therapy that has won FDA approval as a treatment for DMD. The first patient was a 16-year-old young man who died in March. At the time, Sarepta said it would update its prescribing information for Elevidys. “We are deeply saddened by the loss of a second patient and extend our heartfelt condolences to the patient’s family and his care team during this incredibly difficult time,” Louise Rodino-Klapac, PhD, Sareota’s CSO and head of research & development, said in a statement. Sarepta said its temporary suspension of shipments would apply to non-ambulatory patients, and would remain in effect while an enhanced immunosuppressive regimen is evaluated, discussed with regulatory bodies, and put in place...

|
Scooped by
Juan Lama
June 12, 11:38 AM
|
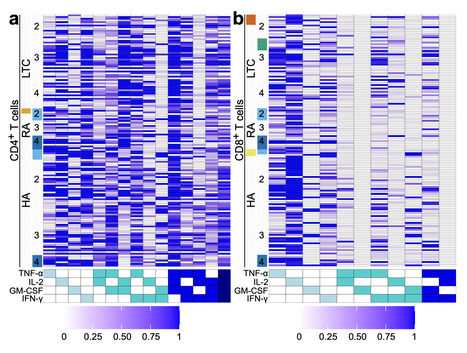
Frequent SARS-CoV-2 vaccination in vulnerable populations has raised concerns that this may contribute to T cell exhaustion, which could negatively affect the quality of immune protection. Herein, we examined the impact of repeated SARS-CoV-2 vaccination on T cell phenotypic and functional exhaustion in frail older adults in long-term care (n = 23), The researchers conclude that repeated SARS-CoV-2 vaccinations do not increase T cell exhaustion or lead to substantive alterations in the T cell compartment in frail older adults in LTC, immunosuppressed individuals with RA, or healthy adults. #COVID19 (n = 10), and healthy adults (n = 43), in Canada. Spike-specific CD4+ and CD8+ T cell levels did not decline in any cohort following repeated SARS-CoV-2 vaccination, nor did the expression of exhaustion markers on spike-specific or total T cells increase. T cell production of multiple cytokines (i.e. polyfunctionality) in response to the spike protein of SARS-CoV-2 did not decline in any cohort following repeated vaccination. None of the cohorts displayed elevated levels of terminally differentiated T cells following multiple SARS-CoV-2 vaccinations. Thus, repeated SARS-CoV-2 vaccination was not associated with increased T cell exhaustion in older frail adults, immunosuppressed individuals, or healthy adults. Repeated vaccination is needed to maintain high levels of SARS-CoV-2 immunity in vulnerable populations, but there is concern that it could lead to immune exhaustion. Here, the authors assess the evidence for immune exhaustion following multiple SARS-CoV-2 vaccination three vulnerable population cohorts in Canada. Published in Nat. Comm. (June 5, 2025): https://doi.org/10.1038/s41467-025-60216-3

|
Scooped by
Juan Lama
June 12, 11:04 AM
|
Just two days after retiring the entirety of the US Centers for Disease Control and Prevention’s vaccine advisory panel, US Health and Human Services Secretary Robert F. Kennedy Jr. has appointed several prominent critics of the government’s Covid-19 response to that committee. He announced eight new members of the CDC’s Advisory Committee on Immunization Practices, or ACIP, on Wednesday. Kennedy had said Monday that the previous 17-member panel that makes recommendations on who should get vaccines and when was rife with conflicts of interest and that he would appoint new “highly credentialed” experts in time for the panel’s June 25 meeting, at which the members are expected to discuss guidance for Covid-19 and HPV shots, among others. In a statement Wednesday, Kennedy said the reassembled panel will demand “definitive safety and efficacy data before making any new vaccine recommendations” but will review data for the current vaccine schedule, as well. The eight new ACIP members include Dr. Robert Malone, a biochemist who made early innovations in the field of messenger RNA but in more recent years has been a vocal critic of mRNA technology in Covid-19 vaccines. The CDC recently narrowed its recommendations for mRNA Covid-19 shots, but some advocates in the Make America Healthy Again movement have pressed Kennedy to go further and bar the vaccines entirely. Another new member is Dr. Martin Kulldorff, a biostatistician and epidemiologist who co-authored an October 2020 strategy on herd immunity known as the Great Barrington Declaration with Dr. Jay Bhattacharya, now director of the US National Institutes of Health. Both Malone’s and Kulldorff’s names were circulated early in the second Trump administration as potential advisers on ACIP or other panels, according to a person familiar with the process who requested anonymity because they weren’t authorized to speak with CNN.....Among the new ACIP members is Dr. Robert Malone, a biochemist who contributed to the mRNA and DNA delivery research early on his career. More recently he has contributed to the spread of misinformation on COVID-19 vaccines.

|
Scooped by
Juan Lama
June 10, 11:28 AM
|
US Health and Human Services Secretary Robert F. Kennedy Jr. on Monday dismissed an expert panel of vaccine advisers that has historically guided the federal government’s vaccine recommendations, saying the group is “plagued with conflicts of interest.” The entirety of the 17-member Advisory Committee on Immunization Practices, which advises the US Centers for Disease Control and Prevention on the vaccine schedule and required coverage of immunizations, will be retired and replaced with new members, Kennedy announced in a Wall Street Journal op-ed. The HHS secretary has authority to appoint and dismiss ACIP members, who typically serve four-year cycles. But removing the entire panel prematurely is unprecedented. Kennedy said that a number of the panel’s members — traditionally pediatricians, epidemiologists, immunologists and other physicians — were “last-minute appointees” of the Biden administration. “Without removing the current members, the current Trump administration would not have been able to appoint a majority of new members until 2028,” he wrote. ACIP members are not political appointees. However Kennedy, a longtime critic of federal vaccine policy and vaccine safety, argued that the current group is rife with conflicts of interest. ACIP had recently published details on conflicts and disclosures for its members from 2000 through 2024. Kennedy also said ACIP not been transparent in its vaccine recommendations. The committee recently considered narrowing the recommendations for Covid-19 vaccinations among children. Kennedy announced last week that the vaccine schedule was updated — without ACIP’s input. One just-dismissed ACIP member told CNN they did not receive a termination notice until after Kennedy’s op-ed published. “I’ve never seen anything this damaging to public health happen in my lifetime,” the adviser said. “I’m shocked. It’s pretty brazen. This will fundamentally destabilize vaccination in America.” The adviser also said that that ACIP “has the most rigorous conflict of interest policy of any organization that I know of.” “Kennedy knows better,” the adviser said. Kennedy had previously pledged to Sen. Bill Cassidy, a Republican from Louisiana and chairman of the Health, Education, Labor and Pensions Committee, that he would consult with the senator on filling key roles on vaccine advisory boards, and that he would maintain the ACIP without changes, according to a February speech Cassidy delivered when he voted in favor of Kennedy’s confirmation as HHS secretary. “Of course, now the fear is that the ACIP will be filled up with people who know nothing about vaccines except suspicion,” Cassidy said in a post on X on Monday. “I’ve just spoken with Secretary Kennedy, and I’ll continue to talk with him to ensure this is not the case.” After CNN’s Manu Raju asked Cassidy how he feels now about his previous support for Kennedy’s nomination, a staff member for the senator interrupted and referred to the statement. Pressed again on whether he regrets voting for Kennedy, Cassidy answered, “I know you’re asking a different question, but you’re trying to get at something similar and I’m just not going to comment on that.” Asked earlier to expand on his conversation with Kennedy on Monday, Cassidy said, “I’d rather not.” Kennedy’s move “raises serious questions,” Republican Sen. Susan Collins of Maine said Monday. “It seems to me to be excessive to ask for everybody’s resignation.” Committee to hold June meeting The CDC committee is scheduled to meet on June 25-27 to discuss vaccinations for Covid-19, RSV, influenza, HPV and meningococcal disease. HHS said the meeting will still take place, giving the agency roughly two weeks to fill its advisory panel. “Appointing people this fast means they were not properly vetted, and there is no real time to check conflict of interests issues,” Dorit Reiss, a professor of law at UC Law San Francisco, told CNN. “This will not restore trust in vaccines, and is not designed to do so.” Other health leaders and institutions quickly spoke in defense of the CDC’s advisory committee. Dr. Richard Besser, president and CEO of the Robert Wood Johnson Foundation and former acting director of the CDC, said the committee had guided US health agencies for more than 60 years and he had relied on its advice during his own 30-year career as a pediatrician. “This decision will make it far more difficult for pediatricians and other providers to care for their patients. The idea that ACIP has failed to scrutinize vaccines being given to pregnant women and babies is absolutely absurd,” Besser said. “Nobody has done more than Secretary Kennedy to sow unwarranted doubt about the safety and effectiveness of vaccines, and this decision demonstrates a complete lack of caring about the health and safety of every American.” Dr. Bruce A. Scott, president of the American Medical Association, said in a statement that Kennedy’s decision undermines trust and “upends a transparent process that has saved countless lives. With an ongoing measles outbreak and routine child vaccination rates declining, this move will further fuel the spread of vaccine-preventable illnesses.” Dr. Susan Kressly, president of the American Academy of Pediatrics, said in a statement that the firings, against the backdrop of contradictory announcements by the Trump Administration in recent days about vaccines, will cause even more confusion and uncertainty for families. “This move undermines the trust pediatricians have built over decades with our patients and leaves us without critical scientific expertise we rely on,” Kressly said. Dr. Tina Tan, president of the Infectious Diseases Society of America, said the committee members were highly qualified and Kennedy’s allegations about the integrity of ACIP were “completely unfounded and will have a significant negative impact on Americans of all ages.” “Unilaterally removing an entire panel of experts is reckless, shortsighted and severely harmful.” The recently dismissed CDC adviser said providers are no longer going to rely on the agency vaccination schedule, and will need to create a parallel committee to ACIP that they can trust. One effort, the Vaccine Integrity Project, launched earlier this year due to concerns that US health leadership was casting unfounded doubt on the safety of well-studied vaccines. Among the project’s considerations: whether there’s a need for a new independent body to evaluate vaccine safety and effectiveness. “The firing of the ACIP represents one of the darkest days in modern public health,” Dr. Mike Osterholm, the Vaccine Integrity Project founder said. “Mr. Kennedy has no interest in science or saving lives. We have entered into a dangerous time for the health of the country.”

|
Scooped by
Juan Lama
June 8, 1:43 PM
|
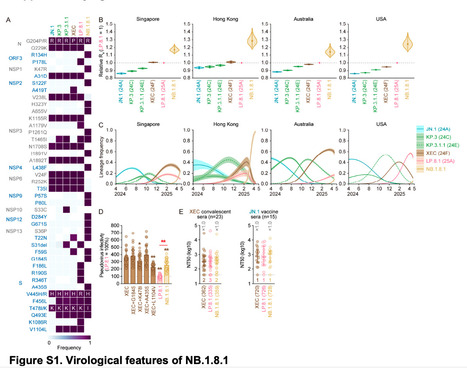
After the spread of SARS-CoV-2 JN.1, its subvariants, such as KP.3 (JN.1.11.1.3) and KP.3.1.1 (JN.1.11.1.3.1.1), and XEC (a recombinant lineage of two JN.1 subvariants), emerged and rapidly spread globally. Subsequently, LP.8.1 (JN.1.11.1.1.1.3.8.1), a descendant lineage of KP.1.1.3 (JN.1.11.1.1.1.3), accounts for approximately 30% of all global infections as of April, 2025, as per data from Nextstrain. Thereafter, NB.1.8.1 (XDV.1.5.1.1.8.1), a descendant lineage of XDV, has started to spread worldwide. XDV is a recombinant lineage of XDE (a recombinant lineage of GW.5.1 [XBB.1.19.1.5.1] and FL.13.4 [XBB.1.9.1.13.4]) and JN.1. NB.1.8.1 has acquired seven spike substitutions and 23 non-spike substitutions compared with JN.1. Compared with the XEC spike protein, the NB.1.8.1 spike bears four substitutions: G184S, K478I, A435S, and L1104V. We estimated the relative effective reproduction number (Re) of NB.1.8.1 using a Bayesian multinomial logistic model based on genome surveillance data from Singapore, Hong Kong, Australia, and the USA, where this variant has spread as of April, 2025 . The Re of NB.1.8.1 was 1·17-fold higher than that of LP.8.1 in Singapore, suggesting its potential to outcompete the other major SARS-CoV-2 lineages. We then assessed the properties of NB.1.8.1 by virological experiments. A lentivirus-based pseudovirus assay showed that the infectivity of NB.1.8.1 was significantly higher (2·5-fold; p<0·0001) than that of LP.8.1. Among the substitutions detected in the NB.1.8.1 spike, K478I and L1104V significantly decreased the infectivity of the XEC pseudovirus. A neutralisation assay using convalescent sera after XEC infection and vaccine sera with the JN.1 mRNA vaccine showed that the 50% neutralisation titre is similar among XEC, LP.8.1, and NB.1.8.1 variants. Overall, although NB.1.8.1 does not show enhanced humoral immune evasion ability compared with LP.8.1, NB.1.8.1 showed higher pseudovirus infectivity than LP.8.1. In line with our data, a previous study showed that the receptor binding domain of NB.1.8.1 binds more strongly to human ACE2 receptor than that of LP.8.1. Therefore, it is plausible to assume that the increased fitness of NB.1.8.1 is due to its augmented infectivity rather than its increased immune evasion. However, in a Chinese cohort, NB.1.8.1 evaded the humoral immunity induced by BA.5 and JN.1 infections more efficiently than LB.8.1. Given that NB.1.8.1 has more than 20 substitutions in non-spike regions compared with LP.8.1 and XEC, it would be warranted to further investigate the virological features of NB.1.8.1 using live isolated virus. Published June 6, 2025 (The Lancet Inf. Dis.):

|
Scooped by
Juan Lama
June 6, 11:35 AM
|
The technology that powered Covid vaccines may also lead scientists to a cure for H.I.V. Using mRNA, Australian researchers said they were able to trick the virus to come out of hiding, a crucial step in ridding the body of it entirely. The research, published last week in Nature Communications, is still preliminary, and so far, has been shown to be successful only in a lab. But it suggests that mRNA has potential far beyond its use in vaccines as a means to deliver therapies against stubborn adversaries. Short for messenger RNA, mRNA is a set of instructions for a gene. In the case of Covid vaccines, the instructions were for a piece of the coronavirus. In the new study, they are for molecules key to targeting H.I.V. Dr. Sharon Lewin, director of the Cumming Global Center for Pandemic Therapeutics in Melbourne, who led the study, called mRNA a “miraculous” tool “to deliver things that you want into places that were not possible before.” Vaccines deploying mRNA instruct the body to produce a fragment of the virus, which then sets off the body’s immune response. In the United States, the shots were initially hailed for turning back the pandemic, then viewed by some with suspicion and fear. Some officials, including Health Secretary Robert F. Kennedy Jr., have falsely said that they are highly dangerous and even deadly. Last week, the Department of Health and Human Services sought to limit the vaccine’s availability to pregnant women, children and healthy younger adults. The administration also canceled a nearly $600 million contract with the drugmaker Moderna to develop an mRNA shot for humans against bird flu. “The fear right now is not rational,” Dr. Lewin said. “mRNA vaccines have been given to millions of people around the world, so we have a very good understanding of their risks.” The new study describes the use of mRNA as a tool to flush H.I.V. out of its hiding places. Other uses could involve providing proteins missing from those with certain diseases or correcting genetic errors. Frauke Muecksch, a virologist at Heidelberg University in Germany who was not involved in the work, called mRNA a “promising, absolutely powerful technology.” Although most people may have only heard of mRNA’s use in science during the pandemic, scientists have been working with it for more than 20 years, she said. “I think it’s not just therapeutically very powerful, but also for basic science, for research, it opens up a lot of avenues,” she added. Potent antiretroviral drugs can now control H.I.V., suppressing it to undetectable levels. Still, minute amounts of the virus lie dormant in so-called reservoirs, waiting for an opportunity to resurge. A cure for H.I.V. would involve ferreting out all of this virus and destroying it, a strategy that has been called “shock and kill.” A significant hurdle is that the virus lies dormant in a particular type of immune cell, called a resting CD4 cell. Because these cells are inactive, they tend to be unresponsive to drugs. The few drugs scientists have previously used to rouse the virus in these cells were not specific to H.I.V. and had unwanted side effects. Research published in Nat. Comm. (May 29, 2025): https://doi.org/10.1038/s41467-025-60001-2

|
Scooped by
Juan Lama
June 4, 12:42 PM
|
Hepatitis B and C Viruses Linked to Increased Risk of Multiple Myeloma: New Meta-Analysis Reveals Crucial Insights A groundbreaking systematic review and meta-analysis published in BMC Cancer has unveiled compelling evidence linking chronic infections with hepatitis B (HBV) and hepatitis C (HCV) viruses to an elevated risk of developing multiple myeloma (MM), a complex hematological malignancy. This comprehensive study synthesizes data spanning over three decades of epidemiological research to clarify the nature of this association, which has previously remained uncertain. Multiple myeloma is characterized by the clonal proliferation of malignant plasma cells within the bone marrow, leading to severe clinical manifestations such as bone destruction, anemia, renal impairment, and immune dysfunction. Despite advances in treatment, MM remains largely incurable and is associated with poor long-term survival. The discovery of potential environmental and infectious risk factors is therefore critical to improving early detection and prevention strategies. The international research team conducted an exhaustive literature search across major biomedical databases, including PubMed, Scopus, Embase, Web of Science, and additional repositories, covering studies published from January 1990 to January 2025. The investigators focused exclusively on high-quality cohort and case-control studies that examined the risk of MM in populations infected with HBV and HCV. In total, seventeen studies met the stringent inclusion criteria: one cohort study and sixteen case-control studies. Within this collection, nine studies focused on the relationship between HBV infection and MM risk, whereas fifteen addressed HCV infection. Employing a random-effects model allowed the researchers to account for variability across studies, enhancing the robustness of the pooled relative risk (RR) estimates. The meta-analysis revealed that individuals with HBV infection face a modestly increased risk of developing MM, with a pooled RR of 1.25 (95% confidence interval [CI]: 0.99–1.58). Although this association approached statistical significance, the findings indicated moderate heterogeneity among the included studies, as quantified by an I² value of 56.52%. This measure reflects differences in study populations, designs, or diagnostic methods that could influence the observed effect sizes. In contrast, the association between HCV infection and MM was more pronounced and statistically significant, with a pooled RR of 1.84 (95% CI: 1.27–2.67). This finding highlights a nearly twofold increase in MM risk among people chronically infected with HCV. The relatively stronger association underscores the distinct pathogenic mechanisms by which HCV may contribute to the development of hematological malignancies, potentially through chronic immune stimulation, direct viral oncogenic effects, or induction of systemic inflammation. Subgroup analyses provided further insight into geographical and demographic trends. Notably, both HBV and HCV infections showed a stronger correlation with MM risk in European populations compared to other regions. For HBV, the risk elevation reached an RR of 1.67 (95% CI: 1.05–2.66), while HCV-infected individuals in Europe displayed an RR of 2.27 (95% CI: 1.21–4.25). These geographic disparities may reflect differences in viral genotypes, healthcare infrastructure, screening practices, or genetic susceptibility factors intrinsic to these populations. Importantly, the systematic review employed rigorous methodological quality assessments using the Newcastle-Ottawa Scale (NOS), ensuring that only studies of adequate quality informed the meta-analysis findings. Statistical techniques were also utilized to evaluate potential publication bias, with Egger’s test indicating no significant bias, thereby strengthening the credibility of the results. The pathophysiological mechanisms linking HBV and HCV infections to MM are still under investigation. However, prevailing hypotheses suggest that chronic viral infections elicit persistent antigenic stimulation of B cells, particularly plasma cells, potentially leading to malignant transformation. Additionally, viral proteins may interfere with cellular regulatory pathways, promoting genomic instability and oncogenesis in susceptible individuals. These revelations bear significant clinical implications. They suggest that patients with chronic hepatitis, especially those infected with HCV, should be closely monitored for early signs of hematological malignancies such as multiple myeloma. Enhanced screening protocols and vigilant follow-ups could facilitate timely diagnosis and intervention, potentially improving patient outcomes. Furthermore, this study emphasizes the importance of integrating infectious disease history into oncological risk stratification models. It also highlights the pressing need for further mechanistic studies to unravel the intricate interactions between chronic viral infections and plasma cell neoplasms. The findings also raise public health considerations, particularly in regions with high prevalence of HBV and HCV. Effective vaccination campaigns against HBV and antiviral treatment programs targeting HCV could indirectly reduce the burden of multiple myeloma by minimizing chronic infection rates, thereby averting long-term oncogenic sequelae. In conclusion, this landmark meta-analysis elucidates a critical link between hepatitis B and C virus infections and the risk of developing multiple myeloma. The stronger association observed with HCV infection calls for heightened awareness and proactive clinical management of at-risk populations. These insights pave the way for multidisciplinary strategies combining virology, oncology, and epidemiology to combat the complex interplay between chronic infections and cancer. As the global healthcare community continues to grapple with the multifaceted challenges posed by hepatitis viruses and hematological malignancies, this research offers a timely reminder of the interconnectedness of infectious diseases and cancer biology. Ongoing research efforts must strive to deepen our understanding and translate these findings into improved patient care and preventative public health policies. Subject of Research: Relationship between Hepatitis B (HBV) and Hepatitis C (HCV) virus infections and the risk of developing multiple myeloma (MM). Research published in BMC Cancer (June 4, 2025): https://doi.org/10.1186/s12885-025-14420-5
|





 Your new post is loading...
Your new post is loading...