Your new post is loading...

|
Scooped by
Juan Lama
March 17, 2020 8:21 PM
|
RetroVirox offers a menu of cell-based antiviral services to evaluate experimental therapies and vaccines against coronaviruses, including SARS-CoV-2. The company offers in vitro testing with SARS-CoV-2 spike pseudovirions and with live SARS-CoV-2 viruses to evaluate entry inhibitors, neutralizing antibodies, and antivirals against the novel coronavirus causative agent of COVID-19. Multiple viral strains are available for testing, including the most recent Omicron variants of concern (XBB.1.5, XBB.1.16, XBB.2.3.2, EG.5.1, JN.1, KP.2.3, and KP.3). Influenza hemagglutinin (HA) pseudovirus are also available for the evaluation of antibodies and vaccines against H5N1 avian influenza. Pseudoviruses coated with the viral spike (S) protein of SARS-CoV-2 are also used to recapitulate the mode of entry of the novel coronavirus. Over 50 spike mutant and variants are available as pseudoviruses. These assays can be used for the following purposes: - To determine the neutralizing activity of therapeutic antibodies and antisera
- To test experimental COVID-19 vaccines using antisera from inoculated animals or humans
- To evaluate small-molecule and other entry inhibitors targeting the S viral protein, the ACE-2 viral receptor, or host proteases and other targets involved in SARS-CoV-2 viral entry
Assays with live replicating SARS-CoV-2, and milder forms of seasonal human coronaviruses (hCoV-OC43 and 229E) allow for the evaluation of inhibitors at all stages of the coronavirus life cycle. Additional Information about cell-based antiviral assays offered at RetroVirox is available here. Request additional information by email at info@retrovirox.com

|
Scooped by
Juan Lama
June 5, 1:19 PM
|
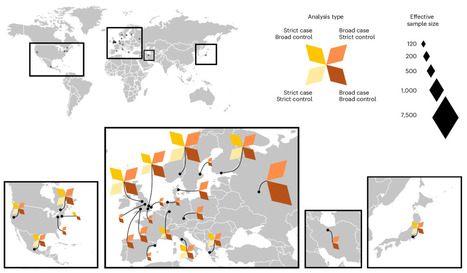
Live-animal markets are a natural laboratory for viruses to evolve and spark deadly outbreaks, yet scientists lack support to study the risks they pose. In the bustling Jatinegara market in Jakarta, cages are stacked three metres high, holding creatures from all corners of Indonesia and beyond. Bats, raccoon dogs, macaques and songbirds — sold as pets or food — are crammed together, their musky odours mingling with the stench of urine and faeces in the damp tropical air. For decades, public-health experts have warned about the risks of infectious diseases jumping from animals to humans in markets such as Jatinegara, which are part of a global industry worth hundreds of billions of dollars annually. These markets remain “the best way of transmitting diseases”, says James Wood, a veterinary epidemiologist at the University of Cambridge, UK. Jatinegara’s location, in an international travel hub with a population of 11 million people, increases that risk considerably. The world is still recovering from the COVID-19 pandemic, which many researchers say probably started, or was at least amplified, at a market selling live animals in Wuhan, China. Yet the wildlife trade still thrives in many parts of the globe. China banned the farming and trading of most wildlife species for food in 2020, but these practices have simply gone underground. “We are back to business as usual,” says Vincent Nijman, a conservation biologist at Oxford Brookes University, UK, with “millions and millions of animals being traded on a daily basis”. The wildlife trade acts as a vast global network of unregulated natural laboratories, through which potential pathogens freely circulate, evolve and ultimately congregate in urban centres, says Andrew Cunningham, a wildlife epidemiologist at the Institute of Zoology in London. “It’s the scariest thing we are doing,” he says. Before the pandemic, there was a strong emphasis on identifying new viruses in the wild. These efforts were driven by the idea that it might be possible to predict which viruses could spark major disease outbreaks. But many researchers now say that this assumption was flawed. Increasingly, scientists are looking to human–wildlife interfaces — including markets and the trade networks that supply them — as crucial sites for the study of zoonoses, human diseases caused by pathogens that normally infect other species. A handful of research groups are now working to understand how pathogens jump between species, why some jumps cause outbreaks and others don’t, and what kinds of intervention can reduce the risks. But such work is costly, can be dangerous and demands sustained support, which has become increasingly hard to secure. Without investing in such research, “you’re really flying blind”, says Maria Van Kerkhove, head of the emerging diseases and zoonoses unit at the World Health Organization in Geneva, Switzerland. “You’re making recommendations that may not be the most appropriate.”....

|
Scooped by
Juan Lama
June 4, 12:25 PM
|
Tracking measles through wastewater is giving health officials a new window into where the virus is spreading. Measles cases in the U.S. have been rare in recent decades, thanks to a strong childhood vaccination program. But a few cases inevitably pop up each year as travelers bring the virus in from other countries and infect unvaccinated people, primarily children. Those cases are no longer blips. Now that the measles vaccination rate is dropping precipitously across the U.S.—due in part to anti-vaccine sentiments—cases are rising. So far in 2025, 14 outbreaks have been reported in 33 states, according to the U.S. Centers for Disease Control and Prevention (CDC). (By comparison, in 2024, there were just 16 outbreaks reported during the entire year.) Scientists may now have a new way to catch cases. For the first time, researchers have posted national information on where the measles virus is showing up in wastewater. Wastewater surveillance is a useful public-health tool because it provides an objective glimpse into where a given virus is causing infections—often before traditional testing methods. For viruses like measles, which infected people shed in urine, feces, or saliva, it can provide a critical heads-up for health officials. “It gives us a finger-to-the-wind weather map of what is happening with infectious diseases,” says Dr. Marlene Wolfe, assistant professor at Emory and principal investigator and co-program director of WastewaterSCAN, an academic and commercial group that includes researchers from Stanford University, Emory University, and Verily (which is Alphabet Inc.'s research organization). Adding measles to the menu of wastewater tests WastewaterSCAN began testing sewage in the U.S. for the COVID-19 virus in 2020 and has since added other disease-causing microbes including influenza, RSV, human metapneumovirus (HMPV), norovirus, enterovirus, mpox, Candida auris, and hepatitis A. This spring, the scientists began developing a test for picking up signs of the active or "wild type" measles virus that is causing outbreaks in the U.S. The test uses samples from nearly 150 sewage sites across the country and can pick up signs of measles within 48 hours. So far, they have detected it in three sites: one day in Hollywood, Md.; four days toward the end of May in Sacramento; and twice in mid-May in Stamford, Conn. WastewaterSCAN is only one such wastewater surveillance network in the country, though it's the first to test for measles on a national level. The CDC launched its National Wastewater Surveillance System in 2020 and includes more than 1,400 sites that cover 150 million Americans. Some state health departments—including in Texas, where the current measles outbreaks began—also test wastewater samples. WastewaterSCAN collaborates with the CDC, but the CDC does not yet test for measles in wastewater. Why wastewater monitoring may be especially useful for measles Doctors who see patients with measles must report the case to the CDC so the agency can track it and respond to any outbreaks. But it takes time for people to develop symptoms, seek medical care, get tested, and then have their case reported if the test is positive. In addition, the first symptoms of measles are common ones like fever, runny nose, and cough—before the telltale rash appears. Plus, not everyone may get sick enough to know they have measles, so their cases may go unreported. Still others may get sick but not have access to health care and therefore never seek medical help. Wastewater monitoring bypasses those hurdles and can theoretically catch evidence of the virus much earlier. That could be especially helpful with a highly contagious disease like measles, in which one infected person can quickly spread the virus to as many as 18 others. “This is anonymous testing that combines everyone in a community,” says Wolfe. “And for measles, it’s likely that shedding [of the virus] into wastewater is happening before people necessarily recognize what they have.” How wastewater data can help health officials This type of tracking for measles is too new to know yet whether it can detect cases in a region before people start testing positive, Wolfe says. But wastewater surveillance was able to pick up signs of the COVID-19 virus days or sometimes even weeks before cases began appearing in hospitals and testing labs. Over time, as more data are collected on measles, health experts can begin to look for trends and patterns, such as increases in the number of positive samples. That could help public-health departments focus resources like vaccines in areas where infections are spreading. If the number of positive samples start to decline, that information could also give them a heads up when infections are under control. What to do if cases are detected near you Getting vaccinated is the best way to protect yourself against measles infection, and the immunization provides close to lifelong immunity. Making sure that anyone who is eligible is up to date on their measles vaccines can help them avoid infection.

|
Scooped by
Juan Lama
June 2, 12:39 PM
|
IAV-NS1 proteins that interact with Cleavage and polyadenylation specific factor 30 are known to inhibit host gene expression. Here we report that in both transfection and infection experiments, a strong attenuation of reporter gene expression is observed when NS1 proteins are fused to protein domains guiding NS1 exclusively to nuclear speckles (NSP). NS1 proteins that are fused to domains that guide them to nuclear or non-nuclear compartments other than NSP show little or no ability to attenuate reporter gene expression. An NSP-localized NS1-effector domain is sufficient to inhibit gene expression. The protein SON is an essential component of NSP. SiRNA-mediated suppression of SON reduced the ability of NS1 to suppress the expression of a reporter gene relative to cells with fully functional NSP. Lastly, we demonstrate that the NS1-mediated suppression relies on transcriptional inhibition. Our data suggest that IAV-NS1 suppresses NSP-promoted gene expression by inhibition of transcription. Published in NPJ Viruses (May 30, 2025): https://doi.org/10.1038/s44298-025-00124-x

|
Scooped by
Juan Lama
May 29, 11:59 AM
|
An HHS spokesperson said that after a comprehensive internal review, the agency had determined that the project did not meet the scientific standards or safety expectations required for continued federal investment. Bird flu has infected 70 people, most of them farm workers, over the past year as it has spread aggressively among cattle herds and poultry flocks. Health Secretary Robert F. Kennedy Jr. has questioned the use of vaccines and earlier this year drew censure from some in the U.S. Congress after he suggested in a television interview that poultry farmers should let the bird flu spread unchecked through their flocks to study chickens who did not contract it. Moderna said it plans to explore alternatives for late-stage development and manufacturing of the vaccine. The company has been banking on revenue from newer mRNA shots, including its bird flu vaccine and experimental COVID-flu combination vaccine, to make up for waning post-pandemic demand for its COVID vaccine. Moderna also said on Wednesday that it had received positive interim data from a mid-stage trial set up to test the safety and immunogenicity of its bird flu vaccine targeting the H5 avian influenza virus subtype. Reporting by Patrick Wingrove; Editing by Sandra Maler

|
Scooped by
Juan Lama
May 28, 12:04 PM
|
Experts say illnesses don't seem more severe and more studies are needed to further assess the risk of antibody escape. The World Health Organization (WHO) Technical Advisory Group on Virus Evolution (TAG-VE) on May 23 announced that it has designated NB.1.8.1 as a SARS-CoV-2 variant under monitoring (VUM), noting that, although proportions are growing rapidly, the virus seems only marginally more immune-evasive than the more dominant LP.8.1 sublineage. The experts said NB.1.8.1 is fueling rises in cases and hospitalization in some countries in the WHO Western Pacific region, but there are no reports that illnesses are more severe than those from other circulating variants. NB.1.8.1 clusters with other JN.1 sublineages and descends from XDV.1.5.1. The earliest sample was collected on January 22. So far sequences have been submitted from 22 countries, and, based on limited sequencing data, officials estimate that the virus made up 10.7% of sequences by the end of April, up significantly from 2.5% in the four previous weeks. The prevalence rose in all three WHO regions that regularly report sequences: Western Pacific, Americas, and European. Low risk based on limited evidence TAG-VE said the risk is currently low and that the confidence in the assessment is low, given that only a single study has assessed antigenicity using pseudoviruses with serologic data from two cohorts. It added that more studies are needed to further assess the risk of antibody escape. So far, the evidence doesn't suggest resistance to the antiviral drug nirmaltelvir, which is one of the two components of Paxlovid treatment. On May 15, the WHO's COVID vaccine composition advisory group said JN.1 and KP.2 remain appropriate vaccine antigens, but monovalent LP.8.1—which seems to show more robust neutralization against newer subvariants—is a suitable alternative. The European Medicines Agency preferentially recommended an LP.8.1 component, and the US Food and Drug Administration also said vaccines should target JN.1, preferentially the LP.8.1 component.

|
Scooped by
Juan Lama
May 27, 4:14 PM
|
NIH-funded breakthrough could enable targeted therapies for many neurological disorders. Research teams funded by the National Institutes of Health (NIH) have created a versatile set of gene delivery systems that can reach different neural cell types in the human brain and spinal cord with exceptional accuracy. These delivery systems are a significant step toward future precise gene therapy to the brain that could safely control errant brain activity with high precision. In contrast, current therapies for brain disorders mostly treat only symptoms. The new delivery systems carry genetic material into the brain and spinal cord for targeted use by specific cell types. This platform has the potential to transform how scientists can study neural circuits. It provides researchers with gene delivery systems for various species used in research, without the need for genetically modified, or transgenic, animals. Examples include illuminating fine structures of brain cells with fluorescent proteins and activating or silencing circuits that control behavior and cognition. “Imagine this new platform as a delivery truck dropping off specialized genetic packages in specific cell neighborhoods in the brain and spinal cord,” said John Ngai, Director of the NIH’s Brain Research Through Advancing Innovative Neurotechnologies Initiative, or The BRAIN Initiative. “With these delivery systems, we can now access and manipulate specific cells in the brain and spinal cord – access that was not possible before at this scale.” The new delivery tools, which use a small, stripped-down adeno-associated virus (AAV) to deliver DNA to target cells, can be broadly applied across many species and experimental systems, including small tissue samples removed during human brain surgeries. The delivery systems have been tested, or validated, in intact living systems, which is an important step for introducing new tools for widespread use. The newly published toolkit includes: - Dozens of delivery systems that selectively target key brain cell types, including excitatory neurons, inhibitory interneurons, striatal and cortical subtypes, brain blood vessel cells, and hard-to-reach neurons in the spinal cord that control body movement and are damaged in several neurological diseases, such as amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy
- Computer programs powered by artificial intelligence (AI) that can identify genetic “light switches,” known as enhancers, that turn genes on in specific brain cell types, using data from many different species – cutting considerable time and effort for scientists looking for these genetic switches.
Overall, this collection of research tools will significantly accelerate understanding of the human brain. Importantly, the toolkit enables access to specific brain cell types in the prefrontal cortex, an area that’s critical for decision-making and uniquely human traits. With other tools in the collection, scientists can better study individual cells and communication pathways known to be affected in several neurological diseases. These include seizure disorders, ALS, Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease – as well as various neuropsychiatric conditions. AAV-based treatments are already approved for some conditions, such as spinal muscular atrophy for which a 2016 approval of a gene therapy known as Zolgensma transformed the lives of infants and young children who once faced severe disability or early death. The new collection of gene delivery resources lays the groundwork for more precise treatments that target only affected cells in the brain, spinal cord, or brain blood vessels. The toolkit is available at distribution centers including Addgene(link is external), a global supplier of genetic research tools, and The Allen Institute's Genetic Tools Atlas(link is external). This collection of publications offers researchers standard operating procedures and user guides for these tools. The work is supported by the NIH’s Brain Research Through Advancing Innovative Neurotechnologies® Initiative, or The BRAIN Initiative®. Funding issued less than four years ago launched a large-scale, team-run project to design new molecular tools that can be useful to many research laboratories. The Armamentarium for Precision Brain Cell Access aims to develop precise and reproducible access to cells and circuits in experimental research models of the brain and spinal cord. The large-scale project brings together experts in the field of molecular biology, neuroscience, and artificial intelligence (AI). The eight papers appear in the May 21 issue of the journals Neuron, Cell, Cell Reports, Cell Genomics, and Cell Reports Methods. Research articles published in Neuron (May 21, 2025): https://www.cell.com/consortium/brain-armamentarium

|
Scooped by
Juan Lama
May 23, 11:35 AM
|
China has pledged to give $500 million to the World Health Organization as the country is set to replace the United States as the group’s top state donor, expanding Beijing’s global influence in the wake of Washington’s retreat from international cooperation. Chinese Vice Premier Liu Guozhong told the World Health Assembly that his country is making the contribution to oppose “unilateralism,” a trait Beijing often ascribes to Washington as relations between the two powers deteriorate...

|
Scooped by
Juan Lama
May 22, 12:02 PM
|
Objective A growing body of evidence points to a role for herpesviruses in the development of Alzheimer’s disease (AD) and a reduced risk of AD among patients receiving antiherpetic medications. We investigated the association between herpes simplex virus type 1 (HSV-1) and AD using real-world data (RWD) from USA. Design In a matched case–control study, patients with AD aged ≥50 years diagnosed between 2006 and 2021 were identified from the IQVIA PharMetrics Plus claims database. Controls were matched in a 1:1 ratio with subjects with AD on age, sex, region, database entry year and healthcare visit numbers. Results The study included 344 628 AD case–control pairs. History of HSV-1 diagnosis was present in 1507 (0.44%) patients with AD compared with 823 (0.24%) controls. HSV-1 diagnosis was found to be associated with AD (adjusted OR 1.80; 95% CI 1.65 to 1.96). Patients with HSV-1 who used antiherpetics were less likely to develop AD compared with those who did not use antiherpetics (adjusted HR 0.83, 95% CI 0.74 to 0.92). Conclusions Findings from this large RWD study implicate HSV-1 in the development of AD and highlight antiherpetic therapies as potentially protective for AD and related dementia. Published in BMJ Open (March 12, 2025): https://doi.org/10.1136/bmjopen-2024-093946

|
Scooped by
Juan Lama
May 21, 1:46 PM
|
After discussions with the FDA, Moderna has withdrawn its application for a combination shot, which had demonstrated efficacy in eliciting antibodies in data announced earlier this month. Just two weeks ago, Moderna announced that its combination flu/COVID-19 vaccine outperformed current shots for both diseases. But on Wednesday, the famed biotech withdrew the FDA application for the shot after consultation with the agency. Moderna had filed for a biological license application (BLA) for the vaccine, called mRNA-1083, in late 2024 with an eye toward a late 2025 approval. The company revealed Phase III results in adults 50 years and older in March, before pushing the expected approval date back to 2026 during its Q1 earnings call, citing an expectation for a request for additional data on flu shot efficacy from the FDA. The withdrawal is a setback for Moderna, which had already expected delays for mRNA-1083. In its first quarter earnings report, the company announced that the shot would be deprioritized given “an extended review timeline.” Following news of the withdrawal, Moderna’s shares dropped 4.5% when the markets opened Wednesday, to $26.79 apiece compared to $27.99 at Tuesday’s close. Moderna plans to resubmit the BLA for its combo vaccine, mRNA-1083, later this year when Phase III data from its investigational seasonal flu vaccine, mRNA-1010, become available. Interim data from that trial is expected this summer. mRNA-1083 contains components of mRNA-1010 and components of its second-generation COVID-19 shot, mRNA-1283. Phase III trial data announced earlier this month showed that mRNA-1083 generated as many or more antibodies against most flu strains and the Omicron XBB.1.5 COVID-19 variant in comparison to Moderna’s standalone COVID-19 shot and standard flu shots Fluarix (marketed by GSK for people aged 50 to 64) and Fluzone (from Sanofi for people aged 65 or older, a stronger dose than Fluarix). BioSpace has reached out to Moderna for further comment. The FDA’s regulation of vaccines is in flux. The agency earlier this year missed a deadline for a decision on Novavax’s protein-based COVID-19 vaccine. That vaccine ultimately won approval last week, but only for a subset of older patients and people with underlying conditions. This is largely reflective of new risk-based approval requirements for future COVID-19 candidates introduced Tuesday by FDA Commissioner Marty Makary and CBER Director Vinay Prasad in an editorial published in the New England Journal of Medicine and in an FDA Town Hall. This follows a new requirement announced by the Department of Health and Human Services that all new vaccine trials have a full placebo arm going forward. In a note to investors after the news of HHS’ new requirements, Leerink Partners wrote that “HHS’s judgment appears questionable and risky, in our view.” They added that “placebo-controlled trials are unnecessary and unethical for many populations,” and said “the world will look to other countries’ health authorities as the gold standards for vaccine testing.” Moderna’s main competitor, Pfizer, has submitted applications for two COVID-19 mRNA vaccines for children aged six months to four years and five to 11 years. Pfizer is also conducting a Phase III trial of a shot for children six months to 11 years, as well as a Phase II trial for its own combo flu/COVID-19

|
Scooped by
Juan Lama
May 20, 11:46 AM
|
NEW YORK, May 20 (Reuters) - The U.S. Food and Drug Administration plans to require new clinical trials for approval of annual COVID-19 boosters for healthy Americans under 65, effectively limiting their availability this fall to older adults and those with a higher risk of developing severe illness, FDA leaders said on Tuesday. FDA Commissioner Marty Makary and top U.S. vaccines regulator Vinay Prasad said based on data from tests that measure immune response in patients, they anticipate that the FDA will be able to approve the boosters for adults over the age of 65 years. It would also be available for everyone over the age of six months with one or more risk factors that put them at high risk for severe COVID-19 outcomes, they said in a piece published in the New England Journal of Medicine on Tuesday. But for healthy people between the ages of six months and 64 years, the FDA expects it would need randomized, controlled trials for drugmakers to get approval for annual shots. Prasad and Makary said that saline could be used as placebo in those trials. Vaccine makers have argued that because COVID vaccines have been changed annually to match the circulating strain of the virus, new placebo-controlled trials could delay availability of the shots until after their usefulness has passed. But Prasad and Makary say the studies are needed to provide evidence that annual shots for healthy younger Americans are evidence-based. "We simply don’t know whether a healthy 52-year-old woman with a normal BMI who has had Covid-19 three times and has received six previous doses of a Covid-19 vaccine will benefit from the seventh dose," Prasad and Makary wrote in the piece. "This policy will compel much-needed evidence generation." There are currently three approved vaccines for COVID-19 in the U.S.: messenger RNA-based shots made by Moderna Inc (MRNA.O), opens new tab and by Pfizer (PFE.N), opens new tab and Germany's BioNTech (22UAy.DE), opens new tab, and a protein-based vaccine made by Novavax Inc. (NVAX.O), opens new tab "This is quite reasonable. The really high risk people - people over 65, people with chronic medical conditions - can still get the vaccine," said Dr. David Boulware, an infections disease specialist at the University of Minnesota. Boulware said he believed it was unlikely that vaccine makers will conduct the clinical trials to receive the broader approval. "This is going to be hundreds of millions of dollars, so they're not going to do the trial in a young population because the sample size would be huge to show benefit. I think its unlikely to be done," he said. U.S. Health and Human Services Secretary Robert F. Kennedy Jr. is a vaccine skeptic, who has long sown doubts about the safety and efficacy of the shots. Makary and Prasad have been critical in the past of the approval process and the evidence supporting the necessity of annual COVID-19 shots for many Americans. They said the U.S. policy has been more aggressive than policies followed in Europe, Mexico and Canada. Still, they acknowledged that the 2020 development of the shots were a major scientific, medical, and regulatory accomplishment. They also called the measles-mumps-rubella vaccine a vital immunization and said it had been "clearly established as safe and highly effective." COVID vaccines are significant products for drugmakers, even as uptake has fallen post-pandemic. In 2024, U.S. sales of COVID boosters - sold primarily by Pfizer and Moderna - topped $3.5 billion. Novavax's shot was approved last week after the FDA missed the April 1 target to make a decision on the vaccine. The FDA already limited its use to older adults and people over the age of 12 with conditions that put them at risk due to the illness. According to the U.S. Centers for Disease Control and Prevention, a wide list of conditions constitutes an additional risk, ranging from various illnesses, such as diabetes and heart disease, to behaviors like physical inactivity and substance abuse. Makary and Prasad said that under the new framework, estimates suggest that some 100 million to 200 million Americans would have access to the annual shots. Reporting by Michael Erman, editing by Deepa Babington

|
Scooped by
Juan Lama
May 14, 7:59 PM
|
Successively emerging SARS-CoV-2 variants lead to repeated epidemic surges through escalated fitness (i.e., relative effective reproduction number between variants). Modeling the genotype–fitness relationship enables us to pinpoint the mutations boosting viral fitness and flag high-risk variants immediately after their detection. Here, we present CoVFit, a protein language model adapted from ESM-2, designed to predict variant fitness based solely on spike protein sequences. CoVFit was trained on genotype–fitness data derived from viral genome surveillance and functional mutation assays related to immune evasion. CoVFit successively ranked the fitness of unknown future variants harboring nearly 15 mutations with informative accuracy. CoVFit identified 959 fitness elevation events throughout SARS-CoV-2 evolution until late 2023. Furthermore, we show that CoVFit is applicable for predicting viral evolution through single amino acid mutations. Our study gives insight into the SARS-CoV-2 fitness landscape and provides a tool for efficiently identifying SARS-CoV-2 variants with higher epidemic risk. Ito et al. present CoVFit, an AI model that predicts variant fitness (transmissibility) from spike protein sequences alone. They further demonstrate its utility in forecasting viral evolution via single amino acid mutations. Published in Nat. Comm. (May 13, 2025): https://doi.org/10.1038/s41467-025-59422-w

|
Scooped by
Juan Lama
May 14, 12:14 PM
|
The plight of the ostrich farm has spurred street protests and a social-media campaign, with activists decrying government overreach. A Federal Court judge has denied a bid to prevent the Canadian Food Inspection Agency from culling 400 ostriches on a B.C. farm that was hit by an avian flu outbreak. The court on Tuesday rejected two judicial reviews that were filed by Universal Ostrich Farms after the agency ordered the flock killed in December, then denied a bid for an exemption in January. Katie Pasitney’s parents own the farm and she said after the ruling was released that they plan “to fight this legally as far as we can go.” She said they were inviting supporters to come to the farm in Edgewood in southeastern B.C. and show “kindness, peacefulness and love” in protest of the cull, adding the Canadian Food Inspection Agency had the “full authority … to come in whenever they want.” Pasitney said the agency had given no indication when culling might begin. “They’re not gonna make it a public topic because they don’t want everybody to know. We have thousands of people behind us,” she said. The Canadian Food Inspection Agency did not immediately provide comment on the court ruling Tuesday. The plight of the ostrich farm has spurred street protests and a social-media campaign to “Save Our Ostriches,” with activists decrying government overreach with the agency’s decision to kill the birds. “Beyond the economic loss, the destruction of a long-established ostrich population is also a source of emotional distress, particularly given the decades of work and investment the principals have dedicated to breeding and raising their flock,” Zinn wrote. “I have considerable sympathy for them.” The family that owns Universal Ostrich Farm has said the birds should be saved because they have developed herd immunity to avian flu and could contribute to the fight against the disease, which has resulted in millions of birds being culled in British Columbia and is the subject of worldwide concern about its potential to result in a human pandemic. Zinn’s ruling said “courts generally stay out of scientific debates,” and could only rule on the reasonableness and fairness of the decisions. “Courts must also respect the demonstrated scientific and technical expertise of administrative agencies,” the judgment says. “When Parliament leaves technical or scientific assessments to specialized administrative bodies, it signals that those bodies, not the courts, are best positioned to make judgments on complex, expertise-driven matters.” The owners of the farm said ostriches that survived the flu have recovered and are happy and healthy. But Zinn found the disposal notice and denial of the farm’s exemption happened in December 2024 and January 2025, and the court can’t consider evidence that wasn’t available when those decisions were made. He said the court “would be faulting decision-makers for lacking a crystal ball.” “This court cannot consider ‘new’ evidence, such as the current health status of the ostriches, recent test results or updated scientific developments,” the ruling says. Zinn said the farm and the agency’s competing scientific submissions invited the court to referee “a contest over whose science on the virus in question is ‘better’ and therefore whose preferred animal and public health policy is ‘wiser.”‘ Zinn said the court couldn’t decide such a contest because it would put the decision beyond the scope of a judicial review focused on determining reasonableness, and “effectively make this court an academy of science and an arbiter of truth in immunology and animal and public health.” The competing expert reports submitted in the case, Zinn ruled, had no weight on his findings. Parliament empowered the Canadian Food Inspection Agency to face public-health threats such as avian flu, Zinn said, authorizing it to “act decisively making swift decisions with far-reaching consequences, often under conditions of scientific uncertainty. This is a challenging mandate.” The court ruling notes that the agency’s “mandate is protective rather than punitive,” and provides compensation to owners whose animals are destroyed, up to $3,000 per animal in the case of ostriches. More than 8.7 million birds have been culled in B.C. at hundreds of farms, most of them commercial, since the first outbreak of a highly contagious form of the avian flu occurred in the spring of 2022. Peace River North legislator Jordan Kealy, who supported the attempt to halt the cull, said he was “devastated” by the decision, and the farm was “completely different” to a normal poultry farm that could quickly restock.“My heart goes out to the family and if people want to reach out to the CFIA and let them know, I honestly believe that there could have been an alternative to this scenario rather than culling and killing all the birds, because they’re 30-year-old animals that are not just easily replaced,” he said. B.C. Agriculture and Food Minister Lana Popham said in a statement that while the government’s thoughts were with the owners of Universal Ostrich Farms “in what is a very difficult time for them, we respect the decision of the courts, as well as the jurisdiction of the CFIA.”
|

|
Scooped by
Juan Lama
Today, 11:35 AM
|
The technology that powered Covid vaccines may also lead scientists to a cure for H.I.V. Using mRNA, Australian researchers said they were able to trick the virus to come out of hiding, a crucial step in ridding the body of it entirely. The research, published last week in Nature Communications, is still preliminary, and so far, has been shown to be successful only in a lab. But it suggests that mRNA has potential far beyond its use in vaccines as a means to deliver therapies against stubborn adversaries. Short for messenger RNA, mRNA is a set of instructions for a gene. In the case of Covid vaccines, the instructions were for a piece of the coronavirus. In the new study, they are for molecules key to targeting H.I.V. Dr. Sharon Lewin, director of the Cumming Global Center for Pandemic Therapeutics in Melbourne, who led the study, called mRNA a “miraculous” tool “to deliver things that you want into places that were not possible before.” Vaccines deploying mRNA instruct the body to produce a fragment of the virus, which then sets off the body’s immune response. In the United States, the shots were initially hailed for turning back the pandemic, then viewed by some with suspicion and fear. Some officials, including Health Secretary Robert F. Kennedy Jr., have falsely said that they are highly dangerous and even deadly. Last week, the Department of Health and Human Services sought to limit the vaccine’s availability to pregnant women, children and healthy younger adults. The administration also canceled a nearly $600 million contract with the drugmaker Moderna to develop an mRNA shot for humans against bird flu. “The fear right now is not rational,” Dr. Lewin said. “mRNA vaccines have been given to millions of people around the world, so we have a very good understanding of their risks.” The new study describes the use of mRNA as a tool to flush H.I.V. out of its hiding places. Other uses could involve providing proteins missing from those with certain diseases or correcting genetic errors. Frauke Muecksch, a virologist at Heidelberg University in Germany who was not involved in the work, called mRNA a “promising, absolutely powerful technology.” Although most people may have only heard of mRNA’s use in science during the pandemic, scientists have been working with it for more than 20 years, she said. “I think it’s not just therapeutically very powerful, but also for basic science, for research, it opens up a lot of avenues,” she added. Potent antiretroviral drugs can now control H.I.V., suppressing it to undetectable levels. Still, minute amounts of the virus lie dormant in so-called reservoirs, waiting for an opportunity to resurge. A cure for H.I.V. would involve ferreting out all of this virus and destroying it, a strategy that has been called “shock and kill.” A significant hurdle is that the virus lies dormant in a particular type of immune cell, called a resting CD4 cell. Because these cells are inactive, they tend to be unresponsive to drugs. The few drugs scientists have previously used to rouse the virus in these cells were not specific to H.I.V. and had unwanted side effects. Research published in Nat. Comm. (May 29, 2025): https://doi.org/10.1038/s41467-025-60001-2

|
Scooped by
Juan Lama
June 4, 12:42 PM
|
Hepatitis B and C Viruses Linked to Increased Risk of Multiple Myeloma: New Meta-Analysis Reveals Crucial Insights A groundbreaking systematic review and meta-analysis published in BMC Cancer has unveiled compelling evidence linking chronic infections with hepatitis B (HBV) and hepatitis C (HCV) viruses to an elevated risk of developing multiple myeloma (MM), a complex hematological malignancy. This comprehensive study synthesizes data spanning over three decades of epidemiological research to clarify the nature of this association, which has previously remained uncertain. Multiple myeloma is characterized by the clonal proliferation of malignant plasma cells within the bone marrow, leading to severe clinical manifestations such as bone destruction, anemia, renal impairment, and immune dysfunction. Despite advances in treatment, MM remains largely incurable and is associated with poor long-term survival. The discovery of potential environmental and infectious risk factors is therefore critical to improving early detection and prevention strategies. The international research team conducted an exhaustive literature search across major biomedical databases, including PubMed, Scopus, Embase, Web of Science, and additional repositories, covering studies published from January 1990 to January 2025. The investigators focused exclusively on high-quality cohort and case-control studies that examined the risk of MM in populations infected with HBV and HCV. In total, seventeen studies met the stringent inclusion criteria: one cohort study and sixteen case-control studies. Within this collection, nine studies focused on the relationship between HBV infection and MM risk, whereas fifteen addressed HCV infection. Employing a random-effects model allowed the researchers to account for variability across studies, enhancing the robustness of the pooled relative risk (RR) estimates. The meta-analysis revealed that individuals with HBV infection face a modestly increased risk of developing MM, with a pooled RR of 1.25 (95% confidence interval [CI]: 0.99–1.58). Although this association approached statistical significance, the findings indicated moderate heterogeneity among the included studies, as quantified by an I² value of 56.52%. This measure reflects differences in study populations, designs, or diagnostic methods that could influence the observed effect sizes. In contrast, the association between HCV infection and MM was more pronounced and statistically significant, with a pooled RR of 1.84 (95% CI: 1.27–2.67). This finding highlights a nearly twofold increase in MM risk among people chronically infected with HCV. The relatively stronger association underscores the distinct pathogenic mechanisms by which HCV may contribute to the development of hematological malignancies, potentially through chronic immune stimulation, direct viral oncogenic effects, or induction of systemic inflammation. Subgroup analyses provided further insight into geographical and demographic trends. Notably, both HBV and HCV infections showed a stronger correlation with MM risk in European populations compared to other regions. For HBV, the risk elevation reached an RR of 1.67 (95% CI: 1.05–2.66), while HCV-infected individuals in Europe displayed an RR of 2.27 (95% CI: 1.21–4.25). These geographic disparities may reflect differences in viral genotypes, healthcare infrastructure, screening practices, or genetic susceptibility factors intrinsic to these populations. Importantly, the systematic review employed rigorous methodological quality assessments using the Newcastle-Ottawa Scale (NOS), ensuring that only studies of adequate quality informed the meta-analysis findings. Statistical techniques were also utilized to evaluate potential publication bias, with Egger’s test indicating no significant bias, thereby strengthening the credibility of the results. The pathophysiological mechanisms linking HBV and HCV infections to MM are still under investigation. However, prevailing hypotheses suggest that chronic viral infections elicit persistent antigenic stimulation of B cells, particularly plasma cells, potentially leading to malignant transformation. Additionally, viral proteins may interfere with cellular regulatory pathways, promoting genomic instability and oncogenesis in susceptible individuals. These revelations bear significant clinical implications. They suggest that patients with chronic hepatitis, especially those infected with HCV, should be closely monitored for early signs of hematological malignancies such as multiple myeloma. Enhanced screening protocols and vigilant follow-ups could facilitate timely diagnosis and intervention, potentially improving patient outcomes. Furthermore, this study emphasizes the importance of integrating infectious disease history into oncological risk stratification models. It also highlights the pressing need for further mechanistic studies to unravel the intricate interactions between chronic viral infections and plasma cell neoplasms. The findings also raise public health considerations, particularly in regions with high prevalence of HBV and HCV. Effective vaccination campaigns against HBV and antiviral treatment programs targeting HCV could indirectly reduce the burden of multiple myeloma by minimizing chronic infection rates, thereby averting long-term oncogenic sequelae. In conclusion, this landmark meta-analysis elucidates a critical link between hepatitis B and C virus infections and the risk of developing multiple myeloma. The stronger association observed with HCV infection calls for heightened awareness and proactive clinical management of at-risk populations. These insights pave the way for multidisciplinary strategies combining virology, oncology, and epidemiology to combat the complex interplay between chronic infections and cancer. As the global healthcare community continues to grapple with the multifaceted challenges posed by hepatitis viruses and hematological malignancies, this research offers a timely reminder of the interconnectedness of infectious diseases and cancer biology. Ongoing research efforts must strive to deepen our understanding and translate these findings into improved patient care and preventative public health policies. Subject of Research: Relationship between Hepatitis B (HBV) and Hepatitis C (HCV) virus infections and the risk of developing multiple myeloma (MM). Research published in BMC Cancer (June 4, 2025): https://doi.org/10.1186/s12885-025-14420-5

|
Scooped by
Juan Lama
June 2, 12:59 PM
|
The FDA has approved Moderna’s new Covid-19 vaccine, though it placed restrictions on its use that the company’s existing Covid shot does not currently face. The new vaccine, which will be marketed under the name mNexspike, will not immediately replace Spikevax. A statement from the company said both vaccines will be available on the market for the time being. “The FDA approval of our third product, mNEXSPIKE, adds an important new tool to help protect people at high risk of severe disease from Covid-19,” Moderna CEO Stéphane Bancel said in the statement. “Covid-19 remains a serious public health threat, with more than 47,000 Americans dying from the virus last year alone.” As it did earlier this month with Novavax’s Covid vaccine, the FDA said the new Moderna shot is licensed for use only in people aged 65 and older and people aged 12 to 64 who have at least one medical condition that puts them at increased risk of becoming seriously ill if they contract the SARS-CoV-2 virus. Such conditions include diabetes, chronic obstructive pulmonary disorder or COPD, and obesity. Spikevax’s license allows its use in people aged 12 years and older. Unlike the Novavax decision, in this case the FDA met the agreed-up decision date for ruling on the Moderna application; it was May 31. The Novavax decision came a month and a half after its April 1 due date, with political staff at the agency reportedly raising concerns after FDA career staff recommended approval of the vaccine. The approval is at least a partial victory for the company, given the skepticism about messenger RNA-based vaccines shared by health secretary Robert F. Kennedy Jr. and his political base, and some of the health department’s recent actions. Earlier this week Kennedy’s Department of Health and Human Services notified Moderna that it was canceling contracts valued at $766 million for the company to develop, test, and license prototype vaccines against several influenza subtypes that could potentially trigger a pandemic, including H5N1 bird flu. Asked to explain the decision, HHS communications director Andrew Nixon called the mRNA technology “under-tested” and raised vague concerns about the vaccine platform’s safety record. Billions of doses of mRNA-based vaccines have been administered around the globe since the Pfizer-BioNTech and Moderna Covid vaccines were the first to receive authorization in late 2020, a mere 11 months after the genetic code of the SARS-2 virus was first shared internationally. That rollout involved close scrutiny by numerous regulatory agencies looking for evidence of adverse events that might have been linked to these vaccines. With the exception of some reports of myocarditis — inflammation of the heart muscle — in some recipients, mostly teenage boys, no significant side effects have been reported. (Covid infection can also trigger myocarditis in this demographic. In fact, it happens at a higher rate, and infection-induced cases are typically more severe than those seen post-vaccination.) The next-generation Covid vaccine uses a refined target to generate antibodies against the SARS-CoV-2 virus, which allows for the use of a smaller dose — one fifth — than is used in Spikevax. In a Phase 3 trial testing the new vaccine against the existing one, mNexspike generated higher antibody levels, especially among older adults, the company reported.

|
Scooped by
Juan Lama
May 31, 12:49 PM
|
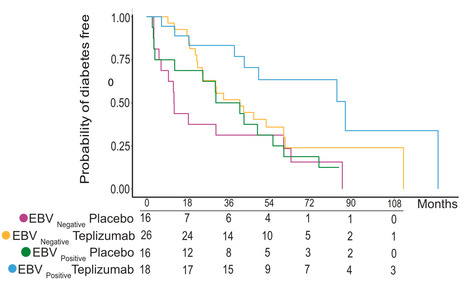
Teplizumab is approved for delaying the diagnosis of type 1 diabetes by modulating progression of disease. Compared to EBV-seronegative patients, those who are EBV-seropositive prior to treatment have a more robust response to teplizumab in two clinical trials. Here we compare the phenotypes, transcriptomes and development of peripheral blood cells before and after teplizumab treatment in participants. Higher number of regulatory T cells and partially exhausted CD8+ T cells are found in EBV-seropositive individuals than in EBV-seronegative controls at the baseline in the TN10 and AbATE trials. Mechanistically, single cell transcriptomics and functional assays identify the downregulation of NFκB and T cell activation pathways after treatment in EBV-seropositive patients; among diabetes antigen-specific CD8+ T cells, T cell receptor and mTOR signaling are also reduced. In parallel, signaling impairment is greater in adaptive than innate immune cells following teplizumab treatment in EBV-seropositive individuals. Our data thus indicate that EBV can impair signaling pathways in immune cells to modulate their responses in the context of type 1 diabetes. Teplizumab is clinically approved for delaying the onset of type 1 diabetes, with the responses affected by EBV serology status. Here the authors pursue immune profiling of EBV+ or EBV- participants before and after teplizumab treatment to find more pronounced immune modulation in treated EBV+ individuals to hint a cellular mechanism for this EBV effect. Published in Nature Comm. (May 30, 2025): https://doi.org/10.1038/s41467-025-60276-5

|
Scooped by
Juan Lama
May 28, 1:47 PM
|
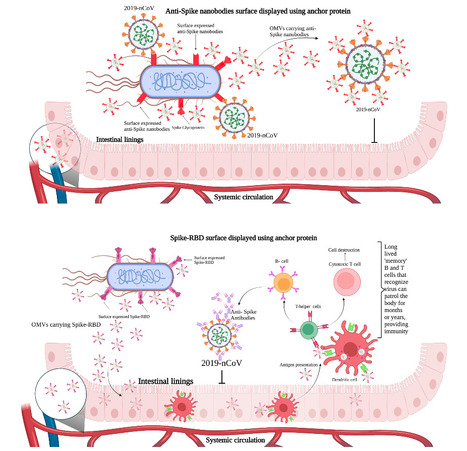
New research from the University of Cincinnati demonstrates how specially engineered bacteria taken orally can operate as a delivery system for antiviral therapies and vaccines. The research, led by Nalinikanth Kotagiri, Ph.D., is published in the journal Gut Microbes. Kotagiri's lab focuses on engineering probiotic bacteria to accomplish a wide variety of functions, from breaking down cancer's defenses to imaging and diagnosing lung infections. A few years ago, the team asked whether the same chassis, using the bacterium E.coli Nissle 1917, could ferry antiviral therapeutic agents or vaccine antigens directly to the gut, a major portal of viral entry. The team focused on the COVID-19 virus, SARS-CoV-2, for the proof-of-concept research. "Oral delivery lets us target the mucosal surfaces where pathogens first gain a foothold while avoiding needles and cold-chain logistics," said Kotagiri, an associate professor in UC's James L. Winkle College of Pharmacy. Vaccine platform Most engineered bacteria keep their therapeutic cargo inside the cell, but vaccines work best when antigens are presented to the immune system. The UC team therefore displayed viral proteins on the bacterial surface and harnessed outer-membrane vesicles (OMVs)—nano-sized spheres that bacteria naturally shed—to act as self-propelled delivery vehicles. Once released, OMVs traffic through the gut epithelium, enter blood circulation and distribute their payload to distant tissues. Nitin S. Kamble, Ph.D., a research scientist in Kotagiri's lab, systematically screened anchor motifs and expression cassettes to optimize antigen density on the probiotic surface. For the vaccine version, the bacteria was designed to express the spike protein found on the surface of the virus that causes COVID-19. This same spike protein is currently delivered through mRNA COVID-19 vaccines. Kotagiri said current vaccines are safe and effective at providing what is called systemic immunity, as antibodies move throughout the whole body in the bloodstream. But there are gateways in the body where viruses typically enter—through mucosal lining in the gastrointestinal system, lungs and other organs—that can be targeted to provide what is called mucosal immunity. In preclinical animal studies, a two-dose oral regimen generated blood-borne (systemic) antibody levels comparable to intramuscular mRNA vaccination. Notably, it produced markedly higher levels of secretory immunoglobulin A (IgA) in the gut and airways—the antibodies that underlie mucosal immunity, considered critical for blocking infection at the point of entry. Therapy platform While vaccines are delivered before a person is infected with a virus, antiviral therapies such as monoclonal antibodies are given as a treatment after infection. The team developed another version of engineered E.coli Nissle 1917 to display therapeutic proteins on the surface. To create a post-exposure therapy, the team encoded anti-spike nanobodies: antibodies that are one-tenth the size of conventional monoclonal antibodies. Although full viral-challenge studies are pending, nanobodies released from the engineered bacteria reached the bloodstream, likely facilitated by OMVs, and accumulated in lung tissue, where they neutralized SARS-CoV-2 in ex-vivo assays. '"A unique aspect of this approach is the use of OMVs as natural postmasters, efficiently packaging and delivering these therapeutic molecules to their intended targets,"' said Kamble. "OMVs can fuse with host cells and deliver a concentrated payload of therapeutic proteins, making them ideal for mucosal delivery." Current IV infusions typically deliver a larger quantity of monoclonal antibodies, but because the probiotic can reside in the gut for days or weeks, it offers a self-renewing and sustained depot of antiviral molecules. Next steps Now that the team has optimized the bacteria for this purpose, Kotagiri has identified an opportunity for quick adaptation of this platform to develop oral vaccines and therapies for common viruses like influenza and norovirus. "All that optimization was necessary for us to prove that this is a platform that we can take forward," he said. "If you have a nanobody or antigens against a virus, we can plug that into our construct." Clinical trials will validate the safety and efficacy of this delivery system for new engineered bacteria targeting other viruses. But Kotagiri said that so far the engineered bacteria have been found to be safe to use and do not generate any adverse immune response or side effects in animal models. Moreover, the parent strain of bacteria has decades of safe use as a probiotic. "In the future, maybe we can integrate both agents so the same bacteria has both the vaccine and the nanobody therapy components," he said. "But the common denominator is the bacteria, where it has the versatility to do both vaccination and therapy, orally." Research cited published in Gut Microbes (May 8, 2025): https://doi.org/10.1080/19490976.2025.2500056

|
Scooped by
Juan Lama
May 27, 4:52 PM
|
Infections can lead to persistent symptoms and diseases such as shingles after varicella zoster or rheumatic fever after streptococcal infections. Similarly, severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infection can result in long coronavirus disease (COVID), typically manifesting as fatigue, pulmonary symptoms and cognitive dysfunction. The biological mechanisms behind long COVID remain unclear. We performed a genome-wide association study for long COVID including up to 6,450 long COVID cases and 1,093,995 population controls from 24 studies across 16 countries. We discovered an association of FOXP4 with long COVID, independent of its previously identified association with severe COVID-19. The signal was replicated in 9,500 long COVID cases and 798,835 population controls. Given the transcription factor FOXP4’s role in lung physiology and pathology, our findings highlight the importance of lung function in the pathophysiology of long COVID. A genome-wide study by the Long COVID Host Genetics Initiative identifies an association between the FOXP4 locus and long COVID, implicating altered lung function in its pathophysiology. Published in Nature Genetics (May 21, 2025): https://doi.org/10.1038/s41588-025-02100-w

|
Scooped by
Juan Lama
May 27, 3:54 PM
|
Health and Human Services Secretary Robert F. Kennedy Jr. announced the change, saying it was based on “common sense” and “good science." The Centers for Disease Control and Prevention no longer recommends routine Covid-19 vaccines for pregnant women and healthy children, Health and Human Services Secretary Robert F. Kennedy Jr.—a noted vaccine skeptic who has pushed conspiracy theories about the shots—announced Tuesday, while a critic said the move puts women and children “in harm's way.” Key Facts - Kennedy, who appeared with National Institutes of Health director Jay Bhattacharya and Food and Drug Administration Martin Makary, said in a video posted to X the Covid vaccine was removed from the CDC’s immunization schedule for pregnant women and healthy children.
- The CDC, whose website has not been updated with the change, previously recommended Covid vaccines for anyone six months and older, pregnant women and women trying to get pregnant.
- The CDC’s website also states there are studies with hundreds of thousands of participants indicating Covid vaccination before and during pregnancy is “safe, effective and beneficial” to the woman and the baby, including research suggesting vaccination builds antibodies that could protect the child.
- Kennedy claimed the Biden administration recommended children receive an additional Covid vaccine “despite the lack of any clinical data to support the repeat booster strategy” among healthy youth.
- Kennedy has faced criticism for his vaccine skepticism, including some opposition during his Senate confirmation hearing earlier this year, during which some Democratic lawmakers questioned Kennedy’s earlier comments that “no vaccine is safe” and his call to pull authorizations for Covid vaccines in May 2021.
- The CDC’s Advisory Committee on Immunization Practices was set to vote on recommendations for the vaccine in June, and the agency’s decision to revoke the advisory follows the FDA adopting a new regulatory framework that would likely narrow recommendations for the shots among people over 65 and those at high risk for severe outcomes.
Surprising Fact Makary and Vinay Prasad, the FDA’s top vaccine regulator, wrote earlier this month that pregnancy was among the underlying medical conditions that probably required additional vaccine shots because of the increased risks of Covid. Chief Critic Paul Offit, a vaccine expert at Children’s Hospital of Pennsylvania, told Forbes Kennedy’s decision to pull the CDC’s vaccine recommendations is “contradictory” and “doesn’t make sense.” Offit cited Makary labeling pregnancy as a high-risk condition for Covid infections, “now they’re saying it’s not, which is it?” Offit said, adding, “All this does is put children in harm’s way and put pregnant women in harm’s way.” Former acting CDC director Richard Besser told NBC in a statement that, while he worked for the agency, there was an “assurance that the recommendations that came to me were based on the best available science and evidence.” The CDC pulling recommendations for healthy children and pregnant women was “clearly not coming from that direction, and that’s greatly concerning,” Besser said. Key Background Talks about adjusting the CDC’s recommendations for Covid vaccines have increased in recent weeks. Jamie Loehr, a member of the CDC’s vaccine advisory panel, said the agency was “seriously considering” adjusting its recommendation language to allow anyone who wanted a vaccine to receive one, CBS reported. Though the CDC’s vaccine panel had reportedly yet to consider dropping recommendations for groups altogether, Kennedy’s Department of Health and Human Services considered pulling advisories earlier this month, people familiar with the agency’s talks told the Wall Street Journal. Kennedy, who now oversees the FDA, CDC and other public health agencies, petitioned the FDA in 2021 to nix emergency authorizations for Covid vaccines relying on mRNA technology, which were recommended for children as young as six months, with approvals for anyone 12 and older.

|
Scooped by
Juan Lama
May 22, 12:28 PM
|
A UK Health Security Agency spokesperson said the risk to the general public is "very low". West Nile virus, which mainly spreads between birds but can also infect people if they're bitten by an infected mosquito, has been detected in UK mosquitoes for the first time, UK health officials say. Although the virus can sometimes make people seriously ill, there is no evidence it is spreading in the UK and the risk to the general public is "very low", the UK Health Security Agency (UKHSA) said. The virus is found in many parts of the world, including Africa, South America and mainland Europe. Climate change and other factors have been pushing mosquitoes - and the diseases they carry - further north in recent years. West Nile virus causes either very minor symptoms or none at all - but around 20% of infected people can experience headaches, high fever and skin issues. In rare cases, it can kill through serious brain illnesses, including encephalitis or meningitis. No specific treatment or vaccines exist for humans. To date, there have been no human cases of West Nile virus acquired in the UK - although, since 2000, there have been seven cases of the disease linked to travel to other countries. West Nile virus is usually present in several regions across the world, including parts of Africa, Asia, South America and Europe, and has expanded in recent years. Research by the UKHSA and the Animal and Plant Health Agency (APHA) found fragments of the virus in mosquitoes collected at ponds near Retford, Nottinghamshire in 2023. "While this is the first detection of West Nile Virus in mosquitoes in the UK so far, it is not unexpected as the virus is already widespread in Europe," said Dr Meera Chand, a deputy director for travel health and infections at UKHSA. Dr Arran Folly, who led the project which found the virus, said its detection is part of a "wider changing landscape, where, in the wake of climate change mosquito-borne diseases are expanding to new areas". While the Aedes vexans mosquito is native to Britain, he added that warming temperatures may bring non-native species to the UK and, with them, the potential of infectious disease. Prof James Logan, from the London School of Hygiene & Tropical Medicine, said the development was "serious" but there was no need for the public to be alarmed. He said surveillance systems were in place to monitor increased mosquito activity and shifting bird migration caused by warmer weather. "But as conditions change, we need to stay one step ahead," he said. "This is a moment to recognise that the UK is no longer immune to some diseases once considered 'tropical'." Prof Logan said the virus is likely to have arrived via an infected bird or mosquito, which can both travel considerable distances during seasonal migration. Infected mosquitoes can transmit the virus to humans, he said, but there was "no evidence of human infection acquired in the UK". "However, the detection of the virus in mosquitoes marks a significant step in that direction," he added. Heather Ferguson, professor of infectious disease ecology at Glasgow University, said several mosquito species native the UK were "known to be capable for transmitting the virus", but do not do so at present because the conditions, such as temperature, "are not favourable". But she said this could change in the future, which is why lots of monitoring, testing and surveillance is always needed. The affected mosquito, one of many thousands of species of mosquito, is often found in wet areas. Experts recommend getting rid of standing water sources where they breed, and taking personal measures such as using mosquito repellent and bed nets. Last year, protests were held in Seville, Spain, after the death of five people infected with the disease.

|
Scooped by
Juan Lama
May 21, 1:59 PM
|
Vaccines for livestock could reduce the risk of human outbreaks, but hurdles remain. As bird flu sweeps across US poultry and cattle farms, researchers are racing to find ways to contain the outbreaks before they ignite a human pandemic. Now, a team of scientists has developed a fresh approach: the first mRNA bird-flu vaccine for cattle. Early findings, posted this month on the preprint server bioRxiv, reveal that the experimental vaccine triggers a strong immune response to the virus, and protects against infection in calves. The results have not yet been peer-reviewed. This development could mark a crucial step towards creating flu vaccines for livestock and reducing the risk of animal-to-human transmission of a virus that poses a “real pandemic threat”, says Scott Hensley, a virologist at the University of Pennsylvania in Philadelphia, and a co-author of the work. Fears of a bird-flu pandemic have been rising since the first confirmed outbreak of the H5N1 avian influenza virus in dairy cattle was reported in March 2024. Since then, the virus has affected more than 1,000 dairy herds across 17 US states. Health officials have linked 64 human infections and one death to the outbreak. A fresh approach To create a cattle vaccine, Hensley and his team built on more than a decade of work on seasonal bird-flu mRNA vaccines. The researchers took one such vaccine candidate and swapped out its viral haemagglutinin gene — which encodes a protein known to elicit an immune response — with the corresponding gene from the new H5N1 virus found on dairy farms. “It’s so easy to switch,” says Hensley. “That’s really the value of using mRNA-based vaccines.” Last year, Hensley’s team showed that their vaccine protects against avian flu in ferrets2, a commonly used laboratory model for testing flu vaccines. For the latest work, they inoculated 10 calves and, 49 days later, fed them milk from H5N1-infected cows — a suspected route of transmission among cattle. After that exposure, the vaccinated calves had significantly lower levels of viral RNA than the unvaccinated calves did, indicating that the vaccine helped to curb infection. The study tested only vaccine responses in calves; much of the avian-flu transmission on dairy farms occurs among lactating adult cattle, says virologist Richard Webby, director of the World Health Organization Collaborating Centre for Studies on the Ecology of Influenza in Animals and Birds in Memphis, Tennessee. Hensley’s team is already working on extra trials in lactating cows. Even without that data, the current results are a strong first step towards developing a vaccine: “It’s good news,“ Webby says.

|
Scooped by
Juan Lama
May 20, 12:11 PM
|
The Food and Drug Administration (FDA) added to a small list of gene-edited animals that can be used in the food supply chain with its approval of a pig produced to resist one of the world’s most costly livestock diseases. The FDA on April 29 announced its approval of the pig made to be resistant to Porcine Reproductive and Respiratory Syndrome (PRRS). The pigs’ genes are edited with CRISPR technology to delete a small portion of their DNA coding, Exon 7 of CD163, which regulates the protein that PRRS viruses use to infect a pig’s cells. The new disease resistant pig breed has been under development for several years by PIC, a subsidiary of Genus, which is a member of the Biotechnology Innovation Organization (BIO). The possibility of preventing PRRS in pigs could be a major victory for pork growers. “PRRS caused an estimated $1.2 billion per year in lost production in the U.S. pork industry from 2016 to 2020, an 80% increase from a decade earlier,” according to an Iowa State University study. Because PRRS also causes bacterial coinfections, it can lead to a 200% increase in use of antibiotics, a study has shown. Overuse of antibiotics in animals is known to drive the rise of antimicrobial resistant superbugs. FDA just beginning to OK gene-edited meat
The pig joins only three other gene-edited animals that are allowed to be sold as food in the U.S.:
- AquaBounty’s AquaAdvantage, an Atlantic salmon produced to grow faster than other salmon, was the first genetically engineered animal for food to receive FDA approval, in 2015.
- GalSafe pigs, genetically engineered to be safe for people with the meat allergy alpha-gal syndrome, were approved by FDA for sale as a food in 2020 but are not widely marketed.
- FDA decided in 2022 that it would not object to Acceligen marketing its “SLICK” beef cattle, which is produced with CRISPR gene editing to have short-hair and thus be more tolerant of hotter climates.
The next step for the PRRS-resistant pig is commercialization. According to Genus, full U.S. commercialization of the PRRS-resistant pigs will be possible once the pigs are approved for sale in U.S. target markets of Mexico, Canada, and Japan. “FDA approval is a fantastic achievement for Genus PIC and represents a major step towards U.S. commercialization. We will now continue to pursue regulatory approvals in other international jurisdictions with a focus on key U.S. export markets” said Jorgen Kokke, Genus’s Chief Executive Officer.

|
Scooped by
Juan Lama
May 19, 12:22 PM
|
The puzzling halt in reported human bird flu cases has prompted health officials to renew calls for vigilance, even as experts grapple with several theories about the sudden drop. While officials urge continued caution, questions linger about potential contributing factors, including the impact of government cuts on case detection, increased fear among immigrant farm workers – a demographic disproportionately affected by the virus – of seeking testing due to immigration enforcement concerns, and the possibility of a natural decline in infections. “We just don't know why there haven't been cases,” said Jennifer Nuzzo, director of the Pandemic Center at Brown University. “I think we should assume there are infections that are occurring in farmworkers that just aren't being detected.” The H5N1 bird flu has been spreading widely among wild birds, poultry and other animals around the world for several years, and starting early last year became a problem in people and cows in the U.S. In the last 14 months, infections have been reported in 70 people in the U.S. — most of them workers on dairy and poultry farms. One person died, but most of the infected people had mild illnesses. The most recent infections confirmed by the Centers for Disease Control and Prevention were in early February in Nevada, Ohio and Wyoming. California had been a hotspot, with three-quarters of the nation’s infections in dairy cattle. But testing and cases among people have fallen off. At least 50 people were tested each month in late 2024, but just three people were tested in March, one in April and none in May so far, state records show. Overall, the state has confirmed H5N1 infections in 38 people, none after Jan. 14. Why bird flu cases are down During a call with U.S. doctors this month, one CDC official noted that there is a seasonality to bird flu: Cases peak in the fall and early winter, possibly due to the migration patterns of wild birds that are primary spreaders of the virus. That could mean the U.S. is experiencing a natural — maybe temporary — decline in cases. It's unlikely that a severe human infection, requiring hospitalization, would go unnoticed, said Michael Osterholm, a University of Minnesota expert on infectious diseases. What's more, a patchwork system that monitors viruses in sewage and wastewater has suggested limited activity recently. New infections are still being detected in birds and cattle, but not as frequently as several months ago. “Given the fact that the number of animal detections has fallen according to USDA data, it’s not surprising that human cases have declined as well,” the CDC said in a statement. Are government cuts affecting bird flu monitoring? Dr. Gregory Gray said he wasn’t concerned about the CDC not identifying new cases in months. “I don’t think that anybody’s hiding anything,” said Gray, an infectious disease speicialist at the University of Texas Medical Branch in Galveston. But Osterholm and some other experts think it's likely that at least some milder infections are going undetected. And they worry that the effort to find them has been eroding. Resignations at the U.S. Department of Agriculture and the Food and Drug Administration’s Center for Veterinary Medicine could slow the government’s bird flu monitoring, said Keith Poulsen, director of the Wisconsin Veterinary Diagnostic Laboratory. Three of 14 experts accepted deferred resignation offers at the National Animal Health Laboratory Network, which responds to disease outbreaks with crucial diagnostic information, he said. They are among more than 15,000 USDA staff to accept the offers, an agency spokesperson said. And dozens of staff were fired at the FDA's Veterinary Laboratory Investigation and Response Network, which investigates animal diseases caused by problems including contaminated pet food. Cats in several states have been sickened and died after eating raw pet food found to contain poultry infected with H5N1. Angela Rasmussen, a virologist at the University of Saskatchewan in Canada, said "targeted surveillance has really dropped off precipitously since Trump took office." She wonders if immigrant farmworkers are too scared to come forward. "I can’t argue with anyone who would be risking getting shipped to a Salvadoran gulag for reporting an exposure or seeking testing,” she said. The CDC characterizes the risk to the general public as low, although it is higher for people who work with cattle and poultry or who are in contact with wild birds. Earlier this month, an agency assessment said there is a “moderate risk” that currently circulating strains of bird flu could cause a future pandemic, but the CDC stressed that other emerging forms of bird flu has been similarly labeled in the past. Still, research is continuing. Texas A&M University scientists have collected blood samples from dairy workers in multiple states to test for signs of past H5N1 exposure, said David Douphrate, a workplace health and safety expert leading the project. The yearlong study is funded by a nearly $4 million grant from the CDC and is expected to conclude in July. Douphrate said he leveraged two decades of relationships with dairy producers and workers to gain access to the farms. “We have had very good participation,” Douphrate said. “They have been very willing.” Similar surveillance is “urgently needed” among domestic cats, said Kristen Coleman, a researcher at the University of Maryland at College Park who studies emerging animal diseases. She recently released a paper reviewing bird flu in infections in cats between 2004 and 2024. Barn cats that died after drinking raw milk were one of the first signs that dairy cows were becoming infected with bird flu in 2024. Since then, the Agriculture Department has confirmed more than 120 domestic cats infected with the virus across the U.S. Infections have mostly been found in cats that died. Less is known about milder infections, whether cats can recover from bird flu — or whether the virus can spill over into people. Coleman has been collecting blood samples from cats across the U.S. to see if they have evidence of previous exposure to the virus. But the process is slow and research funding is uncertain. “It's easy to downplay something because that's usually what humans do,” she said. “But what we really need to be doing is ramping up.”

|
Scooped by
Juan Lama
May 14, 12:35 PM
|
Circular RNAs (circRNAs) are a family of non-coding RNAs that originate from a non-canonical splicing event (backsplicing) that forms covalently closed continuous loops. An analysis of the human immunodeficiency type 1 virus (HIV-1) complex splicing pattern indicated that the virus had the potential to generate at least 15 distinct circRNAs. The predicted HIV circRNAs were amplified utilizing divergent PCR primers and confirmed by RNase R digestion and sequencing. A predictive circRNA-miRNA interaction modeling approach and a series of validation assays determined that two cellular miRNAs, miR-6727-3p and miR-4722-3p, functionally interact with a sequence present in 8 of the HIV circRNAs. Expression of miR-6727-3p and miR-4722-3p restricted HIV-1 replication while a circRNA containing the sequence recognized by miR-6727-3p and miR-4722-3p increased the production of infective virions. Additionally, miR-6727-3p and miR-4722-3p expression was upregulated following HIV-1 infection of primary CD4+ T cells. Overall, the data presented shows that HIV-1 generates circRNAs which promote viral replication by sequestering and inhibiting the functions of miR-6727-3p and miR-4722-3p. Publishes in NPJ Viruses (March 28, 2025): https://doi.org/10.1038/s44298-025-00105-0

|
Scooped by
Juan Lama
May 13, 1:19 PM
|
TUESDAY, May 13, 2025 (HealthDay News) — U.S. health officials are telling travelers aged 60 and older to avoid a chikungunya vaccine while they investigate possible side effects. The U.S. Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) issued the warning late last week. The concern focuses on the Valneva vaccine, known as Ixchiq, The Associated Press reported. Valneva is a specialty vaccine developer based in France. Chikungunya is a tropical illness spread by mosquito bites. It causes fever and painful joints. About 100 to 200 U.S. travelers get the illness each year. The government previously recommended the vaccine for adults traveling to areas where chikungunya may be common, including countries in Central and South America, Asia, India and Africa. The vaccine uses a weakened version of the virus. The AP reported that a panel of CDC advisers recently learned of six seniors who developed serious heart or brain symptoms within a week of vaccination. Most had other health problems. Officials also received reports of more than 10 similar cases in other countries. European regulators are also investigating, AP reported. A CDC advisory panel has now urged caution for adults aged 65 and older considering the Ixchiq vaccine. They also recommended a second vaccine, Vimkunya by Bavarian Nordic, for people 12 and older traveling to areas with chikungunya outbreaks. CDC officials have not yet made a final decision on the new recommendations. SOURCE: The Associated Press, May 12, 2025
|





 Your new post is loading...
Your new post is loading...