 Your new post is loading...
 Your new post is loading...

|
Scooped by
BigField GEG Tech
July 11, 2025 8:59 AM
|
Researchers present a new method to safely and preferentially generate CAR T cells directly inside the body using targeted lipid nanoparticles that deliver mRNA directly to T cells.

|
Scooped by
BigField GEG Tech
July 8, 2024 6:15 AM
|
Researchers have developed a novel version of a key CRISPR gene-editing protein that shows efficient editing activity and is small enough to be packaged within a non-pathogenic virus that can deliver it to target cells.

|
Scooped by
BigField GEG Tech
February 22, 2024 6:45 AM
|
Viruses and virally derived particles have the intrinsic capacity to deliver molecules to cells, but the difficulty of readily altering cell-type selectivity has hindered their use for therapeutic delivery. Here, we show that cell surface marker recognition by antibody fragments displayed on membrane-derived particles encapsulating CRISPR–Cas9 protein and guide RNA can deliver genome editing tools to specific cells. Compared to conventional vectors like adeno-associated virus that rely on evolved capsid tropisms to deliver virally encoded cargo, these Cas9-packaging enveloped delivery vehicles (Cas9-EDVs) leverage predictable antibody–antigen interactions to transiently deliver genome editing machinery selectively to cells of interest. Antibody-targeted Cas9-EDVs preferentially confer genome editing in cognate target cells over bystander cells in mixed populations, both ex vivo and in vivo. By using multiplexed targeting molecules to direct delivery to human T cells, Cas9-EDVs enable the generation of genome-edited chimeric antigen receptor T cells in humanized mice, establishing a programmable delivery modality with the potential for widespread therapeutic utility. Cell-specific molecular delivery with enveloped delivery vehicles enables genome editing ex vivo and in vivo.

|
Scooped by
BigField GEG Tech
December 15, 2023 9:51 AM
|

|
Scooped by
BigField GEG Tech
November 16, 2023 9:56 AM
|
Large genes including several CRISPR-Cas modules like gene activators (CRISPRa) require dual adeno-associated viral (AAV) vectors for an efficient in vivo delivery and expression. Current dual AAV vector approaches have important limitations, e.g., low reconstitution efficiency, production of alien proteins, or low flexibility in split site selection. Here, we present a dual AAV vector technology based on reconstitution via mRNA trans-splicing (REVeRT). REVeRT is flexible in split site selection and can efficiently reconstitute different split genes in numerous in vitro models, in human organoids, and in vivo. Furthermore, REVeRT can functionally reconstitute a CRISPRa module targeting genes in various mouse tissues and organs in single or multiplexed approaches upon different routes of administration. Finally, REVeRT enabled the reconstitution of full-length ABCA4 after intravitreal injection in a mouse model of Stargardt disease. Due to its flexibility and efficiency REVeRT harbors great potential for basic research and clinical applications. Large genes require dual adeno-associated viral (AAV) vectors for in vivo delivery/expression, but current methods have limitations. Here the authors develop and functionally evaluate REVeRT, an efficient and flexible dual AAV vector technology based on reconstitution via mRNA trans-splicing.

|
Scooped by
BigField GEG Tech
June 8, 2023 6:51 AM
|

|
Scooped by
BigField GEG Tech
December 19, 2022 11:00 AM
|

|
Scooped by
BigField GEG Tech
November 25, 2022 9:37 AM
|
FDA approves Hemgenix, an adeno-associated virus vector-based gene therapy indicated for treatment of adults with Hemophilia B (congenital Factor IX deficiency)

|
Scooped by
BigField GEG Tech
November 7, 2022 5:56 AM
|
Irish-Indian company CyGenica is on a mission to solve the huge challenge surrounding targeted intracellular delivery of gene-editing and other therapies. In just five years since the company was founded, the first clinical trials for cancer are in sight. Here, CEO and CSO Dr Nusrat Sanghamitra PhD tells us about how a persona

|
Scooped by
BigField GEG Tech
October 18, 2022 10:15 AM
|
Adeno-associated viruses (AAVs) are the safest and most effective gene delivery vehicles to drive long-term transgene expression in gene therapy. While animal studies have shown promising results, the translatability of AAVs into clinical settings has been partly limited due to their restricted gene packaging capacities, off-target transduction, and immunogenicity. In this study, we analysed over two decades of AAV applications, in 136 clinical trials. This meta-analysis aims to provide an up-to-date overview of the use and successes of AAVs in clinical trials, while evaluating the approaches used to address the above challenges. First, this study reveals that the speed of novel AAV development has varied between therapeutic areas, with particular room for improvement in Central Nervous System disorders, where development has been slow. Second, the lack of dose-dependent toxicity and efficacy data indicates that optimal dosing regimes remain elusive. Third, more clinical data on the effectiveness of various immune-modulation strategies and gene editing approaches are required to direct future research and to accelerate the translation of AAV-mediated gene therapy into human applications.

|
Scooped by
BigField GEG Tech
October 3, 2022 6:58 AM
|
Gene editing has revolutionised research and is set to change the treatment landscape for genetic diseases, with >100 ongoing clinical trials across diverse disease areas. Here, medical doctor Silja Hansen, who is currently a PhD student at Aarhus University in Denmark, discusses the promise, challenges, and future of prime editing t

|
Scooped by
BigField GEG Tech
September 26, 2022 9:44 AM
|
Patients with collagen VI-related disorders experience progressive and debilitating disease. New research findings from Spain’s Institut de Recerca Sant Joan de Déu (IRSJD) provides new hope for these diseases, demonstrating the power of CRISPR-Cas9 to silence a dominant negative mutation in patient fibroblasts.

|
Scooped by
BigField GEG Tech
September 12, 2022 10:45 AM
|
The oncostatic effects of melatonin correlate with increased reactive oxygen species (ROS) levels, but how melatonin induces this ROS generation is unknown. In the present study, we aimed t
|

|
Scooped by
BigField GEG Tech
July 12, 2024 3:04 AM
|
In a new breakthrough, researchers have achieved significant advances in gene editing of lung stem cells, demonstrating the potential for durable genetic correction in mice. A combination of targeted delivery and efficient base editing showcases the transformative potential of CRISPR-based therapies for cystic fibrosis (CF).

|
Scooped by
BigField GEG Tech
March 27, 2024 5:02 AM
|
A newly developed "GPS nanoparticle" injected intravenously can home in on cancer cells to deliver a genetic punch to the protein implicated in tumor growth and spread, according to researchers from Penn State.

|
Scooped by
BigField GEG Tech
January 30, 2024 6:23 AM
|
Prime editing enables precise installation of genomic substitutions, insertions and deletions in living systems. Efficient in vitro and in vivo delivery of prime editing components, however, remains a challenge. Here we report prime editor engineered virus-like particles (PE-eVLPs) that deliver prime editor proteins, prime editing guide RNAs and nicking single guide RNAs as transient ribonucleoprotein complexes. We systematically engineered v3 and v3b PE-eVLPs with 65- to 170-fold higher editing efficiency in human cells compared to a PE-eVLP construct based on our previously reported base editor eVLP architecture. In two mouse models of genetic blindness, single injections of v3 PE-eVLPs resulted in therapeutically relevant levels of prime editing in the retina, protein expression restoration and partial visual function rescue. Optimized PE-eVLPs support transient in vivo delivery of prime editor ribonucleoproteins, enhancing the potential safety of prime editing by reducing off-target editing and obviating the possibility of oncogenic transgene integration. Delivery of prime editors in vivo is improved using virus-like particles.

|
Scooped by
BigField GEG Tech
November 16, 2023 10:36 AM
|
The landmark decision could transform the treatment of sickle-cell disease and beta-thalassaemia — but the technology is expensive.

|
Scooped by
BigField GEG Tech
July 5, 2023 7:22 AM
|
A study in mice reports a CRISPR/Cas9 gene-editing delivery system, capable of bypassing the blood-brain-barrier and modulating neuronal receptor pathways, to treat chronic anxiety.

|
Scooped by
BigField GEG Tech
May 16, 2023 9:20 AM
|
Simple, efficient and well-tolerated delivery of CRISPR genome editing systems into primary cells remains a major challenge. Here we describe an engineered Peptide-Assisted Genome Editing (PAGE) CRISPR–Cas system for rapid and robust editing of primary cells with minimal toxicity. The PAGE system requires only a 30-min incubation with a cell-penetrating Cas9 or Cas12a and a cell-penetrating endosomal escape peptide to achieve robust single and multiplex genome editing. Unlike electroporation-based methods, PAGE gene editing has low cellular toxicity and shows no significant transcriptional perturbation. We demonstrate rapid and efficient editing of primary cells, including human and mouse T cells, as well as human hematopoietic progenitor cells, with editing efficiencies upwards of 98%. PAGE provides a broadly generalizable platform for next-generation genome engineering in primary cells. Peptide-assisted genome editing enables efficient single and multiplex editing in hematopoietic cells.

|
Scooped by
BigField GEG Tech
December 8, 2022 6:46 AM
|
November 18th, 2022. Today, Applied Cells Inc. and GenScript USA Incorporated announced their strategic collaboration to deliver combined cell isolation solutions for cell therapy drug development worldwide. Under this collaboration, GenScript will develop and supply its proprietary research and cGMP grade CytoSinct™ reagents for use in developing CAR-T and other Cell Therapy products on the Applied Cells MARS® Platform.

|
Scooped by
BigField GEG Tech
November 9, 2022 7:26 AM
|

|
Scooped by
BigField GEG Tech
October 20, 2022 5:38 AM
|
A team of researchers at Northwestern University has devised a new platform for gene editing that could inform the future application of a near-limitless library of CRISPR-based therapeutics.

|
Scooped by
BigField GEG Tech
October 6, 2022 5:40 AM
|
In a new paper, University of California, Irvine researchers explain how precision genome editing agents have enabled precise gene correction and disease rescue in inherited retinal diseases (IRDs).

|
Scooped by
BigField GEG Tech
September 27, 2022 6:14 AM
|
A Penn State-led team of interdisciplinary researchers has developed techniques to improve the efficiency of CRISPR-Cas9, the genome editing technique that earned the Nobel Prize in 2020.

|
Scooped by
BigField GEG Tech
September 13, 2022 2:48 AM
|
DNA-based information is a new interdisciplinary field linking information technology and biotechnology.
|




 Your new post is loading...
Your new post is loading...


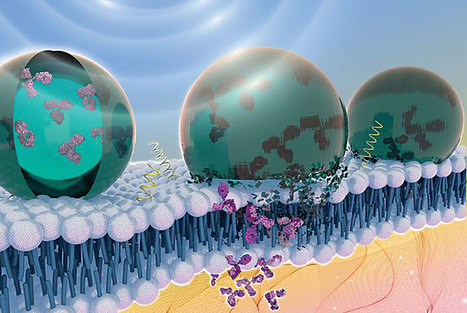


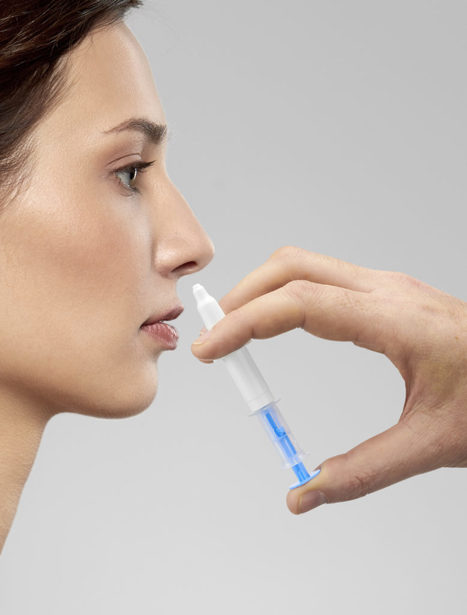








A mouse study reports on a CRISPR/Cas9 gene-editing delivery system, capable of bypassing the blood-brain barrier and modulating neuronal receptor pathways, to treat chronic anxiety. Researchers targeted 5HT-2A, a serotonin receptor known to play a role in anxiety and depression. The authors used a vector based on an inactivated adeno-associated virus to deliver the vector via the nose. The vector delivers a guide RNA to the neurons. The guide RNA binds to the target receptor gene, HTR2A, which is then cleaved at a precise location by the Cas9 enzyme. Five weeks after intranasal administration of the vector and packaging, 75 mice were tested with standard behavioral tests measuring mouse anxiety. For example, anxious mice would choose to spend more time in dark areas and would tend to bury unfamiliar objects such as logs in sawdust rather than leave them alone. Treated mice spent 35.7% more time in light areas than controls and showed a 14.8% decrease in the number of logs buried compared to controls. Mice treated with the CRISPR package showed an 8.47-fold decrease in HTR2A expression in their brains, compared with control mice. According to the authors, non-invasive intranasal administration of CRISPR/Cas9 therapies may therefore help patients with treatment-resistant anxiety.