Genetically engineered mouse models only capture a small fraction of the genetic lesions that drive human cancer. Current CRISPR–Cas9 models can expand this fraction but are limited by their reliance on error-prone DNA repair. Here we develop a system for in vivo prime editing by encoding a Cre-inducible prime editor in the mouse germline. This model allows rapid, precise engineering of a wide range of mutations in cell lines and organoids derived from primary tissues, including a clinically relevant Kras mutation associated with drug resistance and Trp53 hotspot mutations commonly observed in pancreatic cancer. With this system, we demonstrate somatic prime editing in vivo using lipid nanoparticles, and we model lung and pancreatic cancer through viral delivery of prime editing guide RNAs or orthotopic transplantation of prime-edited organoids. We believe that this approach will accelerate functional studies of cancer-associated mutations and complex genetic combinations that are challenging to construct with traditional models. Prime-editing mouse models enable the study of specific cancer mutations in vivo.

|
Scooped by
BigField GEG Tech
onto Animal Models - GEG Tech top picks May 23, 2023 6:43 AM
|




 Your new post is loading...
Your new post is loading...















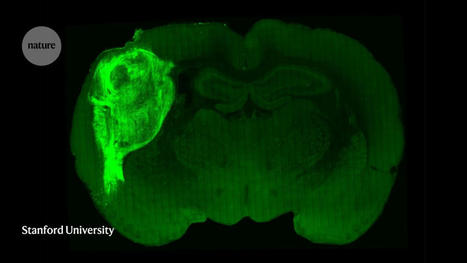







Genomic studies of cancer patients have revealed thousands of mutations linked to tumor development. In an advance that could help scientists make a dent in that long list of unexplored mutations, MIT researchers designed their new mouse models by engineering the gene for the prime editor enzyme into the germline cells of the mice. The encoded prime editor enzyme allows cells to copy an RNA sequence into DNA that is incorporated into the genome. However, the prime editor gene remains silent until activated by the delivery of a specific protein called Cre recombinase. Since the prime editing system is installed in the mouse genome, researchers can initiate tumor growth by injecting Cre recombinase into the tissue where they want a cancer mutation to be expressed, along with a guide RNA that directs Cas9 nickase to make a specific edit in the cells' genome. The RNA guide can be designed to induce single DNA base substitutions, deletions, or additions in a specified gene, allowing the researchers to create any cancer mutation they wish. Using this technique, the researchers have created models of several different mutations of the cancer-causing gene Kras, in different organs. Such models could help researchers identify and test new drugs that target these mutations.