 Your new post is loading...

|
Scooped by
mhryu@live.com
Today, 12:18 PM
|
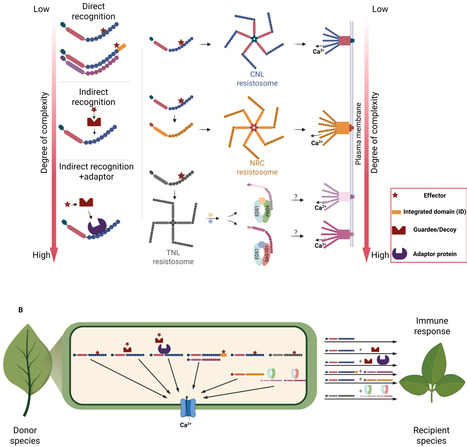
Plants employ cell-surface and intracellular immune receptors to perceive pathogens and activate defense responses. Recent advances in mechanistic understanding of how cell-surface and intracellular immune receptors convert recognition of molecular patterns or effectors into defense activation, combined with the knowledge of receptor repertoire variation both within and between species, allow transfer of immune receptors between species to increase the spectrum of recognition specificities. Here, we summarize recent progress in the functional transfer of immune receptors within and between plant families. We also discuss challenges that limit the transferability of intracellular immune receptors, including the requirement of additional host factors or downstream components and their incompatibility between donor and recipient species. Finally, we provide an overview of future perspectives for bioengineering disease-resistant crops through immune receptor transfer.

|
Scooped by
mhryu@live.com
Today, 12:08 PM
|
The human gut microbiota, particularly the intestinal microbiota, shapes host physiology, disease risk, and therapeutic outcomes through complex metabolic and enzymatic activities. Recent advances in molecular omics, metabolomics, enzyme bioinformatics, and artificial intelligence (AI) have created unprecedented opportunities to elucidate its therapeutic roles to further enable precision microbiome medicine for personalized prevention, diagnosis, and treatment. In this review, we highlight emerging applications that leverage molecular omics and metabolomics technologies to dissect gut microbial functions, along with developments in enzyme bioinformatics and AI tools that reveal gut microbial species, enzymes, and metabolic pathways impacting human health. Finally, we discuss perspectives on data standardization, functional annotation, and interpretability, and how emerging tools are accelerating translational microbiome research.

|
Scooped by
mhryu@live.com
Today, 11:52 AM
|
Lignin, a complex natural aromatic polymer, poses significant challenges to its efficient degradation, hindering the utilization of biomass for many industrial applications. Bacterial degradation of lignin may offer a promising solution to this challenge. This project aimed at elucidating the function of secreted oxidative enzymes from Pseudomonas putida involved in degradation and utilization of lignin and lignin-derived compounds. Using CRISPR-Cas9 and CRISPR-Cas3 systems, the putative lignin-degrading versatile peroxidase gene (VP; PP_1686, originally annotated as glutathione peroxidase GPx) and dye-decolorizing peroxidase gene (PP_3248) were individually knocked out from P. putida KT2440. The ∆PP_1686 mutant exhibited impaired growth and utilization of lignin-derived compounds. This correlated with reduced expression of p-hydroxybenzoate hydroxylase pobA and of DNA repair modules, alongside compensatory upregulation of energy and redox supply pathways. This work expands our knowledge on bacterial glutathione peroxidase by presenting a role beyond ROS scavenging. This work revealed the importance of P. putida VP/GPx in maintaining redox balance while supporting lignin-derived aromatic metabolism, offering new targets for future investigation into stress–metabolism crosstalk and lignin valorization strategies.

|
Scooped by
mhryu@live.com
Today, 11:44 AM
|
RNA detection applications can be augmented if a sensed RNA can be directly functionally transduced. However, there is no generalizable approach that allows an RNA trigger itself to directly activate diverse non-coding RNA effectors. Here, we report engineering of a programmable, RNA trigger-activated, dual-site self-cleaving ribozyme with modular sensing domain and cleavage product. This platform, UNlocked by Activating RNA (UNBAR), is entirely encoded within one RNA strand. The ribozyme can be designed to be almost completely inactive in absence of trigger, and to exhibit single-nucleotide trigger specificity. UNBAR ribozymes carry out cell-free sensing and protein-free amplification of microRNA and viral RNA sequences, and trigger-dependent release of ncRNA effectors sgRNA, shRNA and aptamer. We demonstrate RNA detection and functional transduction by a cleaved aptamer, whose fluorescence can be directly read out as a function of trigger RNA. We further engineer the ribozyme for function in cells, and demonstrate trigger-dependent regulation of CRISPR-Cas9 editing by sgRNA-embedded ribozymes in zebrafish embryos and human cells. UNBAR is a first-in-class modality with potential to be developed into a versatile platform for synthetic biology, diagnostics and gene regulation. To enable a sensed RNA to activate diverse RNA effectors, the authors engineer a programmable dual-site ribozyme that, upon RNA trigger binding, self-cleaves to release an embedded RNA. It enables trigger-dependent release of diverse ncRNAs and controls CRISPR-Cas9 editing in zebrafish and human cells.

|
Scooped by
mhryu@live.com
January 10, 4:46 PM
|
The pathogenic bacterium Vibrio parahaemolyticus represents a substantial economic and public health concern; however, elucidating its virulence mechanisms has been significantly impeded by its inherent resistant to genetic manipulation, primarily attributed to sophisticated immune defense systems including restriction-modification (R-M) modules, CRISPR-Cas systems, standalone DNases, and DdmDE systems. Paradoxically, while genetic modification is essential for overcoming these barriers, the very barriers themselves obstruct DNA introduction. Our investigation focused on the V. parahaemolyticus X1 strain, where initial plasmid transformation attempts proved unsuccessful. However, low-efficiency conjugation allowed knockout of defense genes, thereby silencing the host’s defense mechanisms. Our findings revealed a standalone DNase, Vpn, as the predominant obstacle to foreign DNA entry in the X1 strain, while a DdmDE system executes elimination of invaded plasmids. Leveraging these insights, we created the V. parahaemolyticus X2 strain via sequential depletion of the Vpn nuclease and the DdmDE system. Capitalizing on the bacterium’s exceptional growth rate, characterized by a generation time of approximately 10.5 min, we established a highly efficient molecular cloning platform capable of creating a new plasmid construct within a single day. This work not only presents a strategic framework for genetic manipulation of previously recalcitrant bacterial species but also underscores the potential of fast-growing marine bacteria as promising candidates for next-generation biotechnological applications.

|
Scooped by
mhryu@live.com
January 10, 4:39 PM
|
Single-cell resolution studies have transformed our understanding of microbial systems, revealing substantial cell-to-cell heterogeneity and complex dynamic behaviors. This review describes recent advances in using optogenetics, where light-sensitive proteins control cellular processes, to investigate microbial behavior at the individual cell level. We discuss studies where optogenetic approaches have enabled high-resolution analysis of properties such as relative cell positioning, subcellular localization, morphology, and gene expression dynamics. In addition, we highlight emerging feedback and event-driven control methods that dynamically modulate cellular states using light signals. By leveraging light's unique capabilities for spatial and temporal manipulation, researchers can now probe cellular characteristics with unprecedented precision. We anticipate significant advances as researchers introduce more sophisticated dynamically patterned light signals for single-cell microbial research.

|
Scooped by
mhryu@live.com
January 10, 4:34 PM
|
Magnetotactic bacteria (MTB) utilize magnetosomes to align passively with Earth's magnetic field. Magnetic alignment, coupled with flagellar motility and aerotaxis, enables MTB to perform magneto-aerotaxis-a strategy that constrains their movement to a one-dimensional trajectory along geomagnetic field lines, optimizing their search for low-oxygen niches in aquatic environments. Beyond axially constrained movement, environmental MTB isolates exhibit a hemispherically determined swimming polarity-favoring either magnetic north or south-that has been suggested to facilitate descent into oxygen-depleted zones. However, a direct quantitative evaluation of how matching swimming polarity influences navigation toward low-oxygen environments has remained elusive. Here, we employed microcapillary assays to assess the functional significance of polar magneto-aerotaxis in the model organism Magnetospirillum gryphiswaldense. We found that a magnetic field configuration matching the predominant swimming polarity of the population results in an up to 4-fold increased peak intensity of the aerotactic band compared to populations with non-matching polarity. Competition assays using fluorescently labeled north- and south-seeking populations confirmed that congruence between swimming polarity and magnetic field orientation markedly improves aerotactic band formation in oxygen gradients. Alongside our main findings, we noted biomagnetism-independent phototactic responses integrated with aerotaxis, driving collective unidirectional migration along the oxygen gradient. Our results provide direct evidence that matching swimming polarity with the magnetic field confers a clear advantage in navigating oxygen gradients. These findings reinforce the role of the geomagnetic field in shaping MTB behavior and highlight the adaptive value of magnetotactic swimming polarity in environmental navigation.

|
Scooped by
mhryu@live.com
January 10, 3:58 PM
|
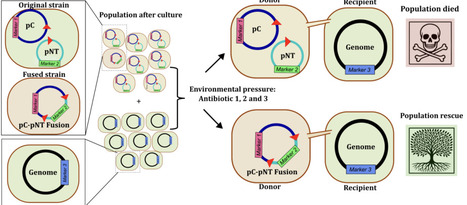
Plasmids are key drivers of bacterial adaptation, yet the mechanisms that generate their diversity remain poorly understood. Here, we show that mobile genetic elements (MGEs) orchestrate a fusion-deletion life cycle that drives the emergence of multireplicon, multidrug-resistant plasmids in Staphylococcus aureus. Large-scale genomic analyses reveal that multireplicon plasmids are widespread and strongly enriched in transposases. Using experimental assays, we demonstrate that rare MGE-mediated fusion events, via homologous recombination or transposition, combine distinct plasmids into single multireplicon elements, expanding gene content and transfer potential. Antibiotic pressure selectively enriches these fused plasmids, rescuing bacterial populations under stress, whereas opposing selective forces, including phage predation, favor deletion derivatives that preserve essential functions and transmissibility. This cyclical process generates dynamic plasmid repertoires with conserved backbones and diverse accessory modules. We propose that MGE-driven fusion-deletion cycles represent a general principle of plasmid evolution, explaining the rapid emergence and persistence of multidrug-resistant plasmids across bacterial pathogens.

|
Scooped by
mhryu@live.com
January 10, 3:41 PM
|
Aptamers are highly selective nucleic acid ligands that overcome many challenges of antibodies, including structural instability and high production costs. The implementation of aptamers into routine research and commercial applications has advanced steadily, though it has been tempered by the limitations of existing discovery methods, which remain relatively slow and low-throughput. SELEX, the gold-standard method for aptamer discovery, has played a pivotal role in the field. As the demand for faster and more tailored solutions grows, there is increasing recognition of the value of newer approaches that can expedite the discovery and optimization of aptamers for specific applications. This review discusses recent advances in the methods used for aptamer engineering, including both wet lab methods performed in vitro, and dry lab methods performed in silico. Applications of engineered aptamers described in recent literature and discuss new developments that could further lead to innovative new applications of aptamers for use both within and outside of the laboratory are discussed. Within the laboratory, these applications include (but are not limited to) target-binding assays and integration into analytical nanomaterials, and outside of the laboratory, diagnostics and biosensing, which will be discussed at length.

|
Scooped by
mhryu@live.com
January 10, 3:19 PM
|
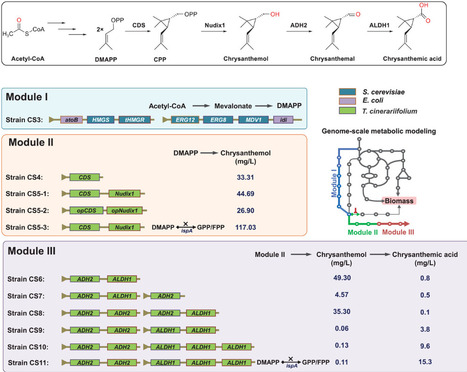
Chrysanthemic acid is an unconventional monoterpene moiety of the natural pesticide pyrethrins with notable anti-insect activity, making its industrial biosynthesis a promising avenue for sustainable agriculture. Here, an E. coli cell factory is designed and build for highly efficient chrysanthemic acid production guided by Genome-scale metabolic models (GEM). The biosynthetic pathway is reconstructed and simulated the metabolic changes caused by exogenous modules are simulated. A key metabolic branch point catalyzed by ispA is identified by this model, and inhibiting its expression using synthetic small RNA redirected the metabolic flux, resulting in the titers of precursor chrysanthemol and chrysanthemic acid increasing by 162% and 59%, respectively. The effect of the expression level of downstream dehydrogenases on chrysanthemic acid titer is also predicated using GEM, and the further optimization of copy number for dehydrogenase genes led to a notably 570% increase in chrysanthemic acid titer experimentally. By integrating the debranching strategy with copy number optimization, a record chrysanthemic acid titer 141.78 mg L−1 is achieved in a bioreactor. The work seamlessly integrated in silico modeling optimization with wet-lab practices that significantly enhance target metabolite titer through metabolic network engineering, offering a new route for constructing efficient cell factories for natural bioproducts.

|
Scooped by
mhryu@live.com
January 10, 3:02 PM
|
Respiratory burst oxidase homolog D (RBOHD)-dependent reactive oxygen species (ROS) in Arabidopsis are well known to suppress pathogen colonization, but their influence on beneficial microbes remains unclear. Here, we found that the beneficial rhizobacterium Pseudomonas anguilliseptica was significantly less enriched in the rhizosphere of rbohD mutants than in that of wild-type plants. Conversely, elevated rhizosphere ROS levels, either triggered by pretreatment with pathogenic Dickeya solani bacteria or caused by mutations in ROS scavenging genes (e.g., in apx1 and cat2 mutants), promoted the rhizosphere recruitment of P. anguilliseptica. This promoting effect was abolished by catalase treatment. In situ microfluidic chemotaxis assays further revealed that P. anguilliseptica exhibits a chemotactic response to low concentrations of hydrogen peroxide ( ≤ 500 nM), accompanied by upregulated expression of chemotaxis- and motility-related genes. Notably, inoculation of P. anguilliseptica effectively suppressed D. solani-induced disease symptoms, and this protective effect was attenuated by catalase treatment. Collectively, these findings reveal a previously unrecognized role of ROS in recruitment beneficial microbiota to enhance plant growth and suppress disease symptoms.

|
Scooped by
mhryu@live.com
January 10, 2:13 PM
|
The apoplast is an important battlefield in plant–pathogen interactions. The late blight oomycete pathogen Phytophthora infestans, for instance, secretes cystatin-like protease inhibitors EpiC1 and EpiC2B to suppress C14, a papain-like immune protease secreted by tomato. Here, we found that P. infestans also secretes two distinct papain-like proteases termed Pain1 and Pain2, which are transcriptionally induced during infection. Both Pains promote P. infestans infection, but not when their catalytic residues are mutated. Strikingly, EpiC1 and EpiC2B preferentially inhibit tomato C14 rather than self-produced Pains, suggesting that they coevolved with Pains to avoid self-inhibition. To mimic the avoidance of inhibition by EpiCs, we engineered C14 (eC14) with seven Pain1 residues that potentially disturb the EpiCs–C14 interface. This eC14 is less sensitive to inhibition by EpiCs and enhances resistance to P. infestans infection. This strategy demonstrates that a pathogen-inspired protein engineering approach can increase crop resistance to plant pathogens.

|
Scooped by
mhryu@live.com
January 10, 1:24 PM
|
Rapid and precise detection of small-molecule metabolites is crucial for optimizing the bioproduction processes. Cell-free systems (CFSs) offer an ideal platform for developing such biosensors due to their speed and suitability for automation. However, transcription factor (TF)-based biosensors, which are key elements for metabolite sensing, suffer from a severe bottleneck in in vitro environments. Their performance is often compromised due to the absence of nucleoid-associated proteins and different DNA topology compared to in vivo conditions. Here, we present a systematic framework for rationally engineering high-performance TF biosensors optimized for CFSs through integrated control of TF availability and promoter sequence. Using an itaconate (ITA)-responsive biosensor regulated by the LysR-type transcriptional regulator ItcR as a model system, we demonstrate that modulating TF supply and redesigning promoter elements substantially enhance sensitivity and dynamic range. Promoter dissection revealed that upstream sequences that function normally in vivo interfered with regulated transcription in CFSs, and that truncation to remove this region restored inducible behavior. Subsequent fine-tuning of the −35 and −10 motifs enhanced RNA polymerase recruitment and regulator interaction, resulting in 19-fold higher maximum signal, a 3.3-fold lower detection limit (0.003 g/L ITA), and a steeper dose-response curve (Hill slope increase from 2.7 to 34.7). The same promoter engineering strategy also improved a 3-hydroxypropionate-responsive biosensor, demonstrating its generality across distinct TF-promoter systems. Collectively, this framework establishes a rational, modular approach for constructing high-performance, topology-aware biosensors in CFSs, directly enabling high-throughput screening and automated biofoundry integration for synthetic biology and metabolic engineering applications.
|

|
Scooped by
mhryu@live.com
Today, 12:15 PM
|
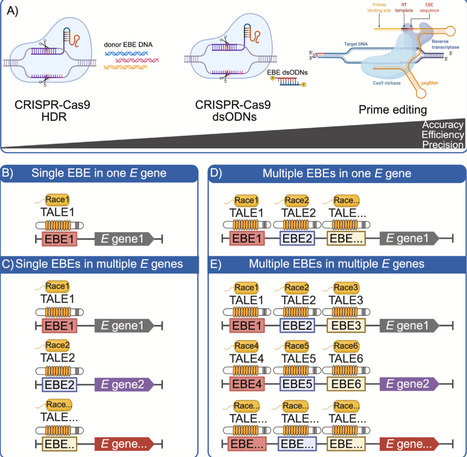
Bacterial type III effector proteins, particularly transcription activator-like effectors (TALEs) secreted by Xanthomonas spp., play critical roles in pathogen–host dynamics. While TALEs facilitate bacterial infections, they also possess vulnerabilities that plants and scientists can exploit to develop mechanisms of resistance. This review encompasses the characteristics and functions of TALEs, examining both their virulence and avirulence roles, and the host plants’ counter-strategies. We highlight advancements in genome editing technologies aimed at combating TALE-dependent plant diseases, with a focus on bacterial blight and leaf streak of rice, but also including bacterial blights of cotton and cassava, and citrus canker. Additionally, we share perspectives on various strategies and approaches for applying genome editing tools to improve disease resistance traits in crop breeding.

|
Scooped by
mhryu@live.com
Today, 12:02 PM
|
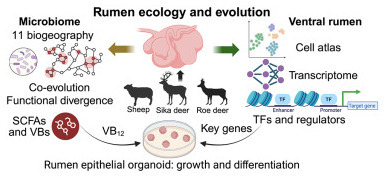
Ruminants thrive in diverse ecosystems by leveraging their rumen microbiome to ferment fibrous plants. However, the spatial biogeography of rumen microbiome and the genetic diversity of the ventral rumen epithelium remain unknown. Here, we present a multi-omics study in roe deer, sika deer, and sheep, integrating region-resolved microbiome and metabolome across 11 ruminal sacs, as well as single-cell RNA sequencing (scRNA-seq), assay for transposase-accessible chromatin using sequencing (ATAC-seq), and bulk RNA sequencing (RNA-seq) of ventral epithelium. We reveal species-specific microbial compositions and metabolic capacities contributing to differences in short-chain fatty acid and vitamin B production. We uncover functional divergence, genomic specialization, and metabolic changes across the microbiome of distinct ruminal sacs. Single-cell profiling reveals changes of immune responses and structural remodeling of the ruminal ventral epithelium. We demonstrate that vitamin B12 promotes epithelial growth and we identify genes enhancing stem cell differentiation. Our results highlight variation in microbial ecology and epithelial architecture among three ruminant species, offering insights to improve livestock productivity.

|
Scooped by
mhryu@live.com
Today, 11:49 AM
|
Dairy industry generates significant effluent streams, comprising wastewaters and nutrient-rich by-products such as cheese whey, which pose environmental challenges for disposal, but also offer valorisation opportunities. Microalgae cultivation on whey represents a sustainable biotechnological approach that couples biomass production with effluent treatment. This study aimed at optimizing microalgal cultivation using ricotta cheese whey as substrate. Eighteen microalgal strains, mainly belonging to Scenedesmus and Chlorella, were initially screened for their ability to grow on whey. Selected strains were further tested in 300-mL photobioreactors under different management options: Low Whey Load (LWL), High Whey Load (HWL), Salinity and Micronutrient (S&M), and Moderate Whey Load (MWL) trials. Results showed that most of the tested strains grew better when cultivated on diluted whey than on standard synthetic media, with increases from 56 to 590% during the first days, and with high removal efficiencies. Several issues were identified during the trials, including nutrient shortage, salinity increase, micronutrients deficiency and potential inhibitory effects of whey compounds, which have hindered long term cultivation of microalgae on whey. In MWL trial, optimization of whey concentration (12% v/v), replenishment strategy (30% of the culture volume every 3-days), and micronutrient supplementation overcame these issues extending the cultivation period and improving biomass yield (203.6 billion cells L-1 whey-1 with the best strain). Overall, this work underscores the importance of carefully optimize microalgal cultivation management to maximise exploitation of dairy by-products and minimize growth-limiting effects of whey accumulation, thus offering a promising starting point for large-scale applications of whey in microalgae cultivation.

|
Scooped by
mhryu@live.com
Today, 11:27 AM
|
Recent advances in high-throughput sequencing, imaging, and phenotyping have carried plant science into the era of ‘big data.’ Complex, multi-scale datasets provide new opportunities to uncover plant molecular mechanisms with a level of detail previously unachievable. Fully exploiting this complexity requires integrating advanced statistics, computational modeling, and artificial intelligence (AI). This mini-review offers guidance on how the combination of AI and mechanistic models is transforming temporal, image-based, and spatial omics data into detailed predictions of robust plant traits. In addition, embedding physical principles into AI models can enhance interpretability and strengthen their biological grounding, leading to more realistic representations of plant inner workings. Together, these advances are reshaping plant science by turning ‘big data’ into deep insights, thus greatly enriching our understanding of plant growth, adaptation, and environmental responses.

|
Scooped by
mhryu@live.com
January 10, 4:43 PM
|
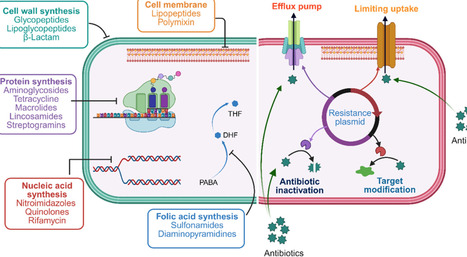
Antibiotics have revolutionized human health by significantly reducing morbidity and mortality associated with bacterial infections. Antibiotics exert bactericidal or bacteriostatic effects through inhibition of cell wall synthesis and disruption of cell membrane integrity, inhibition of protein, nucleic acid synthesis, and other metabolic pathways. Despite their remarkable success since the mid-20th century, antimicrobial resistance (AMR) has emerged as a major global health concern, undermining current treatments and complicating infection management. Key drivers of AMR include the overuse and misuse of antibiotics in clinical settings as well as bacterial adaptations such as genetic mutations and horizontal gene transfer. Mechanistically, these changes can lead to enzymatic inactivation of antibiotics, modification of drug targets, changes in permeability, and active efflux of antimicrobial agents. As resistance rises, antibiotic discovery and development have lagged, creating an urgent need for novel therapeutic strategies and chemical scaffolds. This review examines the antibiotic mechanisms and antibiotic evasion strategies, highlighting genetic and omics approaches used to identify high-priority targets for future drug discovery.

|
Scooped by
mhryu@live.com
January 10, 4:37 PM
|
Bacteria survive hostile conditions in clinically relevant conditions by shutting down protein synthesis, but how they restart growth remains poorly understood. Here, we use an E. coli delta-rimM strain, which exhibits a prolonged growth arrest, as a model to investigate how bacteria recover from this arrested state and restore protein synthesis. RimM is a conserved ribosome maturation factor for the 3'-major (head) domain of the 16S rRNA within the bacterial 30S subunit. The loss of RimM causes a significantly longer delay in recovery than other 30S maturation factors, including RbfA - the presumed primary factor in 30S maturation. Cryo-EM analysis of delta-rimM ribosomes revealed a delayed recruitment of ribosomal proteins to the 30S head domain and increased occupancy of the initiation factors IF1 and IF3, as well as recruitment of the silencing factor RsfS to the 50S subunit. These coordinated changes provide a safeguarding mechanism to block the assembly of premature 70S ribosomes. Notably, while the delayed 30S assembly in delta-rimM reduces the activity of global protein synthesis during the recovery phase, bacteria attempt to compensate for this deficiency by producing higher levels of the ribosomal machinery, indicating a programmatic change in energy allocation to generate the ribosome machinery. These findings highlight the importance of the RimM-assisted assembly of the ribosomal head domain for bacterial recovery from growth arrest.

|
Scooped by
mhryu@live.com
January 10, 4:24 PM
|
Microbial communities often rely on metabolic cooperation, especially under nutrient limitation, but how antibiotics influence these interactions remains unclear. Here, we show that exposure to chloramphenicol, a ribosome-targeting bacteriostatic antibiotic, promotes cooperation in auxotrophic Escherichia coli consortia. Using a proteome-partition model, we find that chloramphenicol-induced growth inhibition elevates levels of the alarmone ppGpp, shifting proteome allocation toward amino acid biosynthesis and export. This regulatory response enhances cross-feeding and supports cooperative growth, even under antibiotic stress. Laboratory experiments confirm that, under low amino acid availability, co-cultures of leucine and phenylalanine auxotrophs display greater tolerance to chloramphenicol than monocultures. Both our theoretical and experimental results reveal a feedback loop in which antibiotic stress strengthens metabolic interdependence, buffering growth inhibition and enhancing community-level tolerance.

|
Scooped by
mhryu@live.com
January 10, 3:57 PM
|
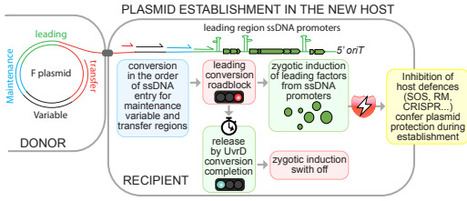
The rapid global spread of antibiotic resistance is mediated by conjugative plasmids, yet how these elements establish immediately after entering new bacterial hosts remains poorly understood. Here we show that plasmids actively coordinate DNA processing with early gene expression to promote their own establishment. Using single-cell measurements of plasmid conversion dynamics, we demonstrate that the F plasmid selectively delays complementary strand synthesis within its leading region, creating a transient single-stranded DNA window that prolongs zygotic induction. This delay is imposed by a single-stranded DNA promoter that forms a structural roadblock to complementary strand DNA synthesis and is resolved by the host helicase UvrD. By extending the single-stranded state of the leading region, plasmids enhance early expression of protective genes that counter host defence responses and promote survival during entry, particularly in non-isogenic hosts. Together, these findings identify early post-entry regulation as a critical determinant of plasmid establishment and reveal how coupling DNA conversion to transient protective gene expression enables conjugative plasmids to overcome host barriers and disseminate antibiotic resistance.

|
Scooped by
mhryu@live.com
January 10, 3:26 PM
|
In recent years, DNA origami technology has advanced rapidly as a groundbreaking method for nanomanufacturing. This technology takes advantage of the unique base-pairing characteristics of DNA, and has significant advantages in constructing spatially ordered and programmable nanostructures. This capability aligns with synthetic biology's core principle of mimicking, extending, and reconstructing natural biological processes by modularly assembling artificial systems. This article provides a comprehensive overview of DNA origami's innovative applications across various domains, including cell membrane surfaces, intercellular communication, intelligent biosensing, and precise gene editing, progressing from the extracellular to the intracellular environment. Finally, this review highlights the synergistic interaction between this technology and cell-free synthetic biology, achieved through the integration of in vitro assembly and cellular regulation, thereby opening new pathways for the rational design of artificial life systems.

|
Scooped by
mhryu@live.com
January 10, 3:15 PM
|
Inspired by the natural ability of bacteriophages to deliver genetic material directly into host cells, we employed a bottom-up approach to construct a multifunctional synthetic DNA origami needle-like structure. This origami is functionalized with trastuzumab antibodies, cholesterol, protective polymers, and two dyes, which together enable selective targeting and insertion into SKBR3 cancer cells. A disulfide-linked dye payload was attached to the apex of the needle, allowing controlled release in the cytoplasm triggered by the high intracellular glutathione concentration. Real-time tracking of the payload confirmed both successful targeting of the origami structure and subsequent direct cytosolic delivery. By mimicking fundamental mechanisms of bacteriophages, we propose that this artificial needle structure can serve as a prototypical device for the targeted delivery of small-molecule drugs directly into the cytosol.

|
Scooped by
mhryu@live.com
January 10, 2:22 PM
|
Adenosine-to-inosine (A-to-I) RNA editing by ADAR enzymes shapes transcript fate and underpins emerging RNA editing therapeutics, yet predicting which adenosines are edited remains difficult. We introduce ADAR-GPT, a model-agnostic fine-tuning framework that adapts a GPT-class language model to classify editing at candidate sites using sequence context in standardized 201 nt windows with the target adenosine explicitly marked. We train and evaluate on GTEx liver data (n=131 samples) at a clinically relevant 15% editing threshold, using a two-stage continual fine-tuning approach where lower thresholds serve as curriculum data to progressively sharpen decision boundaries. Using sequence data, ADAR-GPT demonstrates competitive or superior performance when benchmarked against established computational approaches, including convolutional and foundation model architectures, achieving a better balance of recall, precision, and specificity alongside stronger operating-curve metrics. The approach is reproducible and portable across GPT backbones without architectural changes. Beyond accurate site classification, ADAR-GPT provides practical adenosine scoring to prioritize experimental targets and inform guide RNA design, with a framework adaptable to new datasets and model architectures.

|
Scooped by
mhryu@live.com
January 10, 1:40 PM
|
Microbes play a pivotal role in the Earth’s carbon cycle, regulating greenhouse gas fluxes by emitting, fixing and transforming CO2. Among them, acetogens stand out for their ability to fix CO2 through the Wood–Ljungdahl pathway, an ancient, highly energy-efficient route to acetyl-CoA that operates at thermodynamic limits. By coupling hydrogen (H2) or carbon monoxide oxidation to CO2 fixation, acetogens conserve energy while generating biomass and valuable products such as ethanol and acetate. These features position them as promising microbial cell factories for sustainable bioproduction via gas fermentation. Recent advances in metabolic engineering and synthetic biology have expanded the production spectrum of acetogens, enabling production of platform chemicals at lab-to-commercial scale. Yet, CO2-only bioconversion remains energetically challenging compared to syngas-based applications, requiring innovative solutions in strain development, bioprocess optimisation and integration of renewable energy sources. This review highlights the central role of model acetogens in anaerobic CO2 conversion, covering their metabolic capabilities, strain development and emerging bioprocess strategies to unlock their potential for low-carbon biomanufacturing.
|
 Your new post is loading...
Your new post is loading...




























1str, transcription factors interact with chromatin/DNA in living cells is influenced by the transactivation domains of transcription factors. Traditional models assume that transcription factors first bind DNA through their DNA-binding domains and then use their activation domains to recruit the transcriptional machinery. The study of Fan et al. supports a model in which activation domains can instead bind coactivators that are already bound to DNA, tethering transcription factors indirectly to chromatin.
(G) Proposed model for how the activation domain tethers the synthetic TF to chromatin through the coactivator. This situation occurs at active loci where the coactivator has been recruited by the orange and blue TFs.