 Your new post is loading...

|
Scooped by
?
Today, 11:36 AM
|
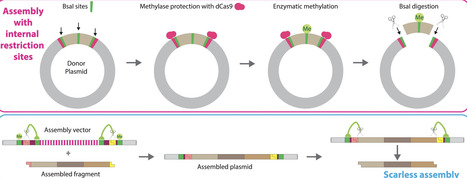
Type IIS restriction enzyme-mediated DNA assembly is efficient but has sequence constraints and can result in unwanted sequence scars. To overcome these drawbacks, we developed UniClo, a type IIS restriction enzyme-mediated method for universal and flexible DNA assembly. This is achieved through a combination of vector engineering, DNA methylation using recombinant methylases, and steric blockade using CRISPR–dCas9 technology to regulate this methylation. Type IIS restriction enzyme sites within fragments to be assembled are methylated using recombinant methylases, while the fragment-flanking outer sites are protected from methylation by a recombinant dCas9–sgRNA complex. During the subsequent assembly reaction, only the protected flanking sites are cut as only they are unmethylated. Fragments are correctly assembled, despite containing internal sites for the single type IIS restriction enzyme used for the one-pot assembly. The assembled plasmid can be used as a donor plasmid in a subsequent assembly round with the same type IIS restriction enzyme and the assembly vector engineering ensures removal of potential scars by a trimming process. This simplifies assembly design and only three vectors are required for any multi-round assembly. These vectors all use the same pair of overhangs. UniClo provides a simple scarless approach for hierarchical assembly of any sequence and has wide potential application.

|
Scooped by
?
Today, 11:20 AM
|
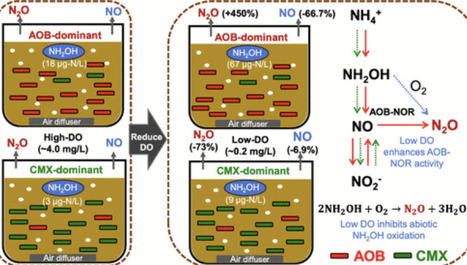
Nitrification significantly contributes to N2O emissions in wastewater treatment, typically enhanced at low dissolved oxygen (DO). The present study revealed that low DO (∼0.2 mg/L) enhanced the N2O emission factor (EF) from nitrification by 4.5 times in canonical ammonia-oxidizing bacteria (AOB)-dominated sludge, while it reduced N2O EF by 73% in comammox Nitrospira-dominated sludge. During nitrification, the accumulation of intermediate NH2OH in AOB-dominant sludge was much higher and increased more significantly by low DO (from 0.018 to 0.067 mg-N/L) compared to comammox Nitrospira-dominant sludge (from 0.004 to 0.009 mg-N/L). In AOB-dominant sludge, the increased NH2OH and upregulated AOB-NOR gene at low DO promoted NO reduction, thus increasing N2O EF while simultaneously decreasing NO emission. In the comammox Nitrospira-dominant sludge, N2O was primarily produced via abiotic pathways. Further investigation found that N2O production decreased significantly under low DO in inactivated sludge and pure water with the sole addition of NH2OH, suggesting that low DO inhibited N2O formation via abiotic NH2OH oxidation. Therefore, in comammox Nitrospira-dominant sludge, low DO decreased N2O production primarily due to the inhibition of low DO to N2O formation via abiotic NH2OH oxidation and the low NH2OH accumulation. These results imply that enriching comammox Nitrospira in the wastewater treatment process can ensure stably low N2O emissions regardless of the variations in DO.

|
Scooped by
?
Today, 11:09 AM
|
Recombinant proteins play a crucial role in both fundamental research and biotechnology. In the laboratory, recombinant proteins are used in a myriad of ways, including to label cells, localize proteins and isolate complexes. In the clinic, antibody-based therapeutics can dramatically increase cancer survival rates, while virus-like particles (VLPs) are being developed as next-generation vaccines. These innovations have escalated demands for biopharmaceutical recombinant proteins. However, in traditional systems (e.g. mammalian and microbial) the expression of recombinant proteins can be prohibitively expensive. One sustainable, low-cost solution is to use a microalgal-based expression system, such as Chlamydomonas reinhardtii, Phaeodactylum tricornutum, Chlorella sp., Haematococcus pluvialis or Nannochloropsis gaditana. Tools for microalgal protein expression are developing rapidly. Yet our understanding of recombinant protein expression and purification in microalgal systems lags that of traditional systems. Here, we review the impact of commonly used affinity and epitope tags (e.g. Polyhistidine-tag, Strep-tag II, HA-tag and FLAG-tag) on recombinant protein detection, purification and biofunctionality in microalgae. Additionally, we review fluorescent protein tags (such as GFP, mVenus, DsRed and mCherry) and protease cleavage sites, including ‘self-cleaving’ 2A peptides. Finally, we provide guidance on experimental design to enhance the likelihood of successfully expressing recombinant proteins in microalgae.

|
Scooped by
?
Today, 10:55 AM
|
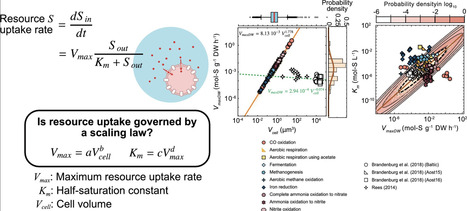
Microbial growth is often described in terms of resource uptake rates, making the understanding and parameterisation of these rate-limiting processes critical for microbial modelling. In phototrophic plankton, theoretical studies suggest that nutrient uptake is limited by mechanistic processes involving membrane transporters, and it has been observed that the cell-specific maximum resource uptake rate (Vmax) follows a power-law relationship with cell size, as well as a trade-off between Vmax and the half-saturation constant (Km). These constraints may also apply to chemotrophic microorganisms; however, many datasets lack direct cell-size measurements. We therefore leveraged the assumption that prokaryotic cell sizes, Vmax, and Km each follow log-normal distributions, drawing parallels with established phytoplankton scaling laws. Our analysis suggests that chemotrophic organisms generally exhibit higher maximum uptake rate per dry weight (VmaxDW) and Km values than phototrophs, and that VmaxDW and Km are not strongly correlated when all chemotroph data are combined. Furthermore, the Bayesian-derived exponents for VmaxDW and Km exceeded those expected from allometric scaling relationships based on the membrane-transport capacity observed for phototrophs, implying that a range of additional factors likely affect observed kinetic parameters.

|
Scooped by
?
Today, 10:35 AM
|
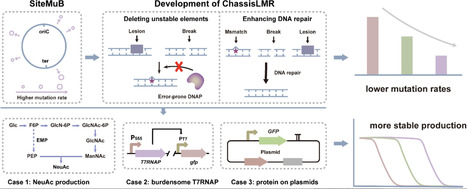
Microbial bioproduction is an important approach to realizing green biomanufacturing. However, poor bioproduction stability caused by genetic heterogeneity is one of the important factors limiting its industrial-scale applications. Here, two methods have been developed to reduce genetic heterogeneity in Bacillus subtilis. SiteMuB (the site-dependent mutation bias) was proposed to enable stable genome integration expression by analyzing the spontaneous mutation rate of the same DNA sequences integrated at different genome sites. Additionally, robustly growing chassis with low mutation rates (ChassisLMR) were developed by deleting unstable elements and enhancing DNA repair. These methods were then employed to improve the production stability of small molecule metabolites and proteins. In N-acetylneuraminic acid production, after 76 generations of cell division, corresponding to the number of cell generations required for > 200-m3 industrial-scale production, strains with SiteMuB and ChassisLMR achieved 15.9-fold and 11.1-fold higher titres than that of the starting strain, respectively. Moreover, by improving the genetic stability of burdensome T7RNAP, combining SiteMuB with ChassisLMR stably maintained the T7 expression system for up to 74 generations, representing a 2.1-fold improvement. Furthermore, ChassisLMR improved the production stability of GFP on the plasmids by 1.38-fold. Overall, SiteMuB and ChassisLMR provide broadly applicable and highly efficient ways to achieve stable bioproduction by reducing genetic heterogeneity.

|
Scooped by
?
Today, 10:24 AM
|
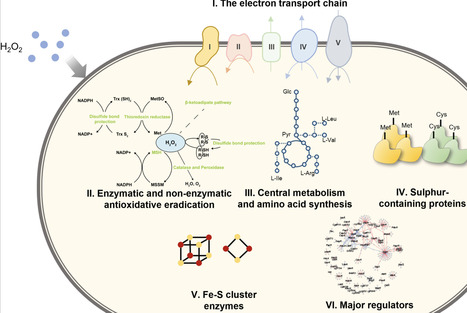
Corynebacterium glutamicum serves as a pivotal industrial chassis for biomanufacturing and an ideal model for studying the phylogenetically related pathogen Mycobacterium tuberculosis. Oxidative stress poses a critical challenge to microorganisms during aerobic industrial processes and immune cell-mediated antibacterial killing by perturbing cellular redox homeostasis, affecting central metabolism, and damaging the integrity of biomacromolecules. However, the intricate mechanisms underlying the dynamic defence of C. glutamicum, despite previous transcriptomic studies on acute and adaptive responses to oxidative stresses, remain largely unclear, hindering strain engineering for industrial applications and the development of effective antimicrobial treatments. In this study, the susceptibility of C. glutamicum to hydrogen peroxide (H2O2) was evaluated, and the inhibitory dynamics of H2O2 were characterized through viable cell counting. RNA sequencing (RNA-seq) was employed to analyse gene expression changes after exposure to 720 mM H2O2. The treatment induced differential expression of 966 and 787 genes at 2 and 6 h, respectively, reflecting perturbations across a broad array of pathways, including (i) enhanced H2O2 and peroxide scavenging, mycothiol biosynthesis, and iron chelation; (ii) repressed central metabolism and enhanced anaplerosis; (iii) elevated sulphur assimilation; (iv) altered amino acid biosynthesis; and (v) altered transcriptional regulation in response to oxidative stress. Further validation by overexpression of ahpD, cysN, and exogenous supplementation with l-methionine and l-cysteine significantly enhanced bacterial tolerance to H2O2. Overall, this study provides the most comprehensive analysis to date of temporal cellular adaptation to H2O2 stress in C. glutamicum, establishing a foundation for future applications in both biomanufacturing and antimicrobial research.

|
Scooped by
?
Today, 10:11 AM
|
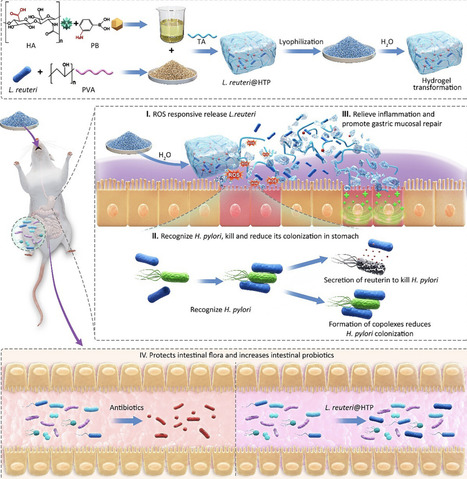
Lactobacillus reuteri (L. reuteri) therapies represent a potentially effective approach to eradicating Helicobacter pylori (H. pylori). However, the difficulty in bacterial viability preservation and harsh gastric environment compromises the survival and on-target delivery of L. reuteri. This study presents a novel bacterium-mediated bacterial elimination strategy using an edible L. reuteri@HTP probiotic powder for targeted bacterial elimination. The probiotic powder is obtained by grinding a lyophilized hydrogel composed of L. reuteri, hyaluronic acid (HA), tannic acid (TA), and polyvinyl alcohol (PVA). Upon contact with water, the powder quickly transforms into a hydrogel, enhancing L. reuteri’s survival in the harsh gastric environment and ensuring selective release at H. pylori-infected inflammatory sites. L. reuteri targets and reduces H. pylori colonization while secreting reuterin to eliminate the bacteria. Additionally, TA's antioxidant properties help alleviate inflammation, and HA supports gastric mucosal repair. L. reuteri@HTP powder preserves the integrity of the gut microbiota, facilitating the restoration of a healthy microbiome. In particular, the probiotic powder remains stable at room temperature for at least six months, providing a promising alternative to traditional antibiotics for H. pylori treatment. This strategy combines targeted eradication, mucosal healing, and microbiome restoration, offering a new approach to treating gastric infections.

|
Scooped by
?
Today, 9:56 AM
|
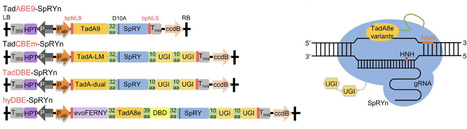
Base editing enables precise nucleotide substitutions within a relatively broad editing window (5–6 nucleotides). However, considerable bystander editing significantly compromise its accuracy. Point mutagenesis, a powerful approach for gradient-tuning protein function, facilitates the generation of diverse plant phenotypes to meet the demands of complex environments and consumer preferences. Here, a series of plant base editors is engineered by fusing three optimized TadA8e variants, TadA9, TadA-LM, and TadA-dual, with a PAM-flexible SpRY nickase (SpRYn, with 5′-NNN PAM recognition). These editors enable A-to-G, C-to-T, and dual-base (simultaneous A-to-G and C-to-T) conversions within a highly condensed active window (1–3 nucleotides). Performance evaluations reveal that the TadDBE (TadA Dual-Base Editor) achieves the most robust outcomes, delivering dual-base editing efficiencies ranging from 2.3% to 61.4%, while maintaining minimal off-target activity. Utilizing TadDBE, targeted point mutagenesis is performed on OsBadh2, a gene encoding betaine aldehyde dehydrogenase that plays a critical role in the biosynthesis of 2-acetyl-1-pyrroline (2-AP), a key aromatic compound. This approach yields rice lines exhibiting gradient-tuned aromatic profiles and optimized levels of 2-AP and γ-aminobutyric acid (GABA). These evolved TadA-derived editors provide a precise, PAM-flexible platform for base editing and represent a versatile strategy for generating genome-edited plants with gradient-tuned agronomic traits.

|
Scooped by
?
Today, 9:31 AM
|
Risk assessment frameworks for plant agricultural biotechnology products have been in place for decades, focused on the evaluation of living biotechnology products created through genetic engineering. These products contain genetic material from outside the breeder’s gene pool, which is often from different taxa or represents “novel combinations of genetic material”. These products are typically considered to be “genetically modified” (GM) organisms in regulatory jurisdictions. However, in the microbial world, particularly among Bacteria and Archaea, the rapid expansion of genome sequence databases shows that natural microbial innovation primarily occurs through the natural exchange of genetic material from various sources, even from different taxa. This means that many microbes can be considered naturally occurring GM organisms. This raises the question of whether labeling a microbe as GM is always scientifically relevant for risk assessment. In most regulatory frameworks, being classified as GM significantly impacts the registration path, especially for microbes intended for environmental release. A more effective and science-based regulatory approach would assess the actual functions of a microbe rather than relying on the uncertain classification of its genetic material. This would benefit regulators, developers, and society by promoting the use of microbial technologies for agricultural use.

|
Scooped by
?
Today, 1:02 AM
|
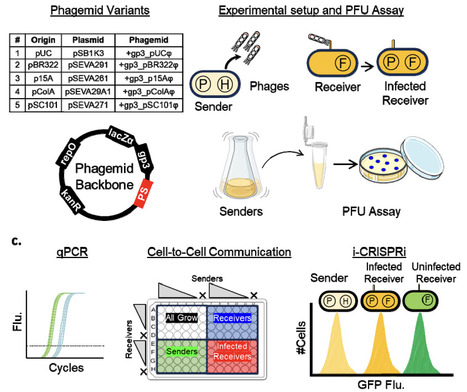
Intercellular communication is essential for distributed genetic circuits operating across cells in multicellular consortia. While diverse signalling molecules have been employed--ranging from quorum sensing signals, secondary metabolites, and pheromones to peptides, and nucleic acids--phage-packaged DNA offers a highly programmable method for communicating information between cells. Here, we present a library of five M13 phagemid variants with distinct replication origins, including those based on the Standard European Vector Architecture (SEVA) family, designed to tune the growth and secretion dynamics of sender strains. We systematically characterize how intracellular phagemid copy number varies with cellular growth physiology and how this, in turn, affects phage secretion rates. In co-cultures, these dynamics influence resource competition and modulate communication outcomes between sender and receiver cells. Leveraging the intercellular CRISPR interference (i-CRISPRi) system, we quantify phagemid transfer frequencies and identify rapid-transfer variants that enable efficient, low-burden communication. The phagemid toolbox developed here expands the repertoire of available phagemids for DNA-payload delivery applications and for implementing intercellular communication in multicellular circuits.

|
Scooped by
?
Today, 12:28 AM
|
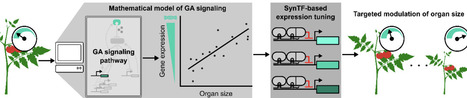
Enhancements to crop morphology, such as the semi-dwarfing that helped drive the green revolution, are often driven by changes in gene dosage. These changes are challenging to translate across varieties and species, which slows the pace of crop improvement. Synthetic transcription factors (SynTFs) offer a rapid alternative to generate targeted alterations to gene dosage. However, the complexity of developmental pathways makes it unclear how to best apply them to predictably engineer morphology. In this work, we explore if mathematical modeling can guide SynTF-based expression modulation of genes in the signaling pathway of the phytohormone, gibberellin (GA), which is a central regulator of cell expansion, to elucidate the design principles for engineering organ size. We demonstrate that modulation of GA signaling gene expression can generate consistent dwarfing across tissues in both controlled and variable environments in the model plant Arabidopsis thaliana, and that the degree of dwarfing is dependent on the strength of regulation as predicted by modeling. We further validate the predictive power of the model by demonstrating its capacity to accurately predict the qualitative impacts of different regulatory architectures for both increasing and decreasing organ size. Finally, we show that these insights can be generalized for engineering organ size in the crop Solanum lycopersicum (tomato). This work creates a framework for predictable engineering of an agriculturally important trait, organ size, across tissues and plant species. It also serves as a proof-of-concept for how mathematical models can guide SynTF-based alterations in gene dosage to enable bottom-up design of plant phenotypes.

|
Scooped by
?
June 22, 10:49 PM
|
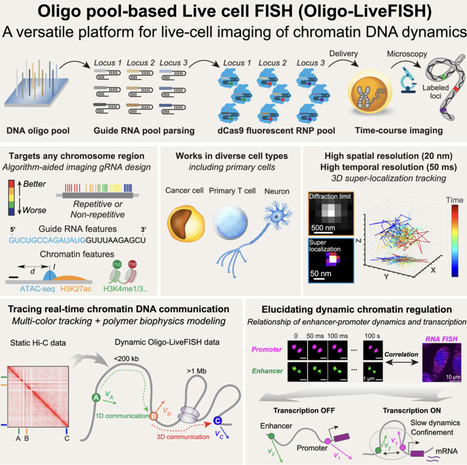
Three-dimensional (3D) genome dynamics are crucial for cellular functions and disease. However, real-time, live-cell DNA visualization remains challenging, as existing methods are often confined to repetitive regions, suffer from low resolution, or require complex genome engineering. Here, we present Oligo-LiveFISH, a high-resolution, reagent-based platform for dynamically tracking non-repetitive genomic loci in diverse cell types, including primary cells. Oligo-LiveFISH utilizes fluorescent guide RNA (gRNA) oligo pools generated by computational design, in vitro transcription, and chemical labeling, delivered as ribonucleoproteins. Utilizing machine learning, we characterized the impact of gRNA design and chromatin features on imaging efficiency. Multi-color Oligo-LiveFISH achieved 20-nm spatial resolution and 50-ms temporal resolution in 3D, capturing real-time enhancer and promoter dynamics. Our measurements and dynamic modeling revealed two distinct modes of chromatin communication, and active transcription slows enhancer-promoter dynamics at endogenous genes like FOS. Oligo-LiveFISH offers a versatile platform for studying 3D genome dynamics and their links to cellular processes and disease.

|
Scooped by
?
June 22, 2:49 PM
|
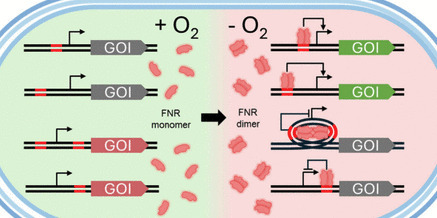
Promoters responsive to changes in cultivation conditions are essential tools for dynamic metabolic engineering. Oxygen-responsive promoters, in particular, exhibit significant application potential in oxygen-limited fermentation processes. However, currently reported oxygen-dependent promoters exhibit limited dynamic ranges, and notably, there remains a lack of research on oxygen-responsive negatively regulated promoters. In this study, we designed and characterized a series of dissolved oxygen-responsive promoters in Escherichia coli under the regulation of the transcription factor fumarate-nitrate reduction (FNR). Anaerobically activated promoters were constructed by inserting FNR binding site (FBS) upstream of inducible core promoters, while anaerobically repressed promoters were developed by inserting FBS downstream of or flanking constitutive promoters. The most effective anaerobically activated promoters showed 24–138-fold higher activity under anaerobic conditions compared to aerobic conditions. Under anaerobic conditions, promoters with DNA looping-mediated anaerobic repression maintained only 8–17% of the activity observed under aerobic conditions. These promoters were specifically regulated by FNR, as confirmed by tests in a DH5α Δfnr strain, and responded rapidly to oxygen depletion (within 30 min). The utility of these genetic tools was demonstrated by applying them to enhance pyruvate production in E. coli. An engineered strain with anaerobic-repressed aceE and anaerobic-activated atpAGD genes produced 5.76 g/L pyruvate at 55.7% yield in shake flask fermentations. This study offers an expanded toolbox of oxygen-responsive promoters that enable precise gene regulation based on dissolved oxygen levels, providing novel genetic strategies for developing efficient two-stage fermentation processes with separated growth and production phases.
|

|
Scooped by
?
Today, 11:30 AM
|
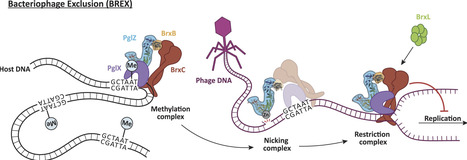
BREX (Bacteriophage Exclusion) systems, identified through shared identity with Pgl (Phage Growth Limitation) systems, are a widespread, highly diverse group of phage defence systems found throughout bacteria and archaea. The varied BREX Types harbor multiple protein subunits (between four and eight) and all encode a conserved putative phosphatase, PglZ, and an equally conserved, putative ATPase, BrxC. Almost all BREX systems also contain a site-specific methyltransferase, PglX. Despite having determined the structure and fundamental biophysical and biochemical behaviours of several BREX factors (including the PglX methyltransferase, the BrxL effector, the BrxA DNA-binding protein, and a commonly-associated transcriptional regulator, BrxR), the mechanism by which BREX impedes phage replication remains largely undetermined. In this study, we identified a stable BREX sub-complex of PglZ:BrxB, generated and validated a structural model of that protein complex, and assessed the biochemical activity of PglZ from BREX, revealing it to be a metal-dependent nuclease. PglZ can cleave cyclic oligonucleotides, linear oligonucleotides, plasmid DNA and both non-modified and modified linear phage genomes. PglZ nuclease activity has no obvious role in BREX-dependent methylation, but does contribute to BREX phage defence. BrxB binding does not impact PglZ nuclease activity. These data contribute to our growing understanding of BREX phage defence.

|
Scooped by
?
Today, 11:12 AM
|
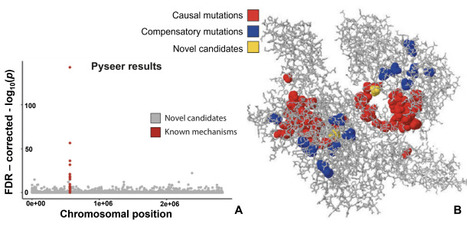
How can we identify causal genetic mechanisms governing bacterial traits? Initial efforts entrusting machine learning models to handle the task of predicting phenotype from genotype yield high accuracy scores. However, attempts to extract meaningful interpretations from the predictive models are found to be corrupted by falsely identified “causal” features. Relying solely on pattern recognition and correlations is unreliable, significantly so in bacterial genomics settings where high-dimensionality and spurious associations are the norm. Though it is not yet clear whether we can overcome this hurdle, significant efforts are being made towards discovering potential high-risk bacterial genetic variants. In view of this, we set up open problems surrounding phenotype prediction from bacterial whole-genome datasets and extending those approaches to learning causal effects, and discuss challenges that impact the reliability of a machine’s decision-making when faced with datasets of this nature. We identify major sources of non-injectivity in the formulation of the genotype-to-phenotype mapping function—linkage-disequilibrium, limited sampling, information loss in representations, unmeasured confounders and observational noise—and analyse their implications for machine learning applications. Using a collection of 4,140 Staphylococcus aureus isolates, we illustrate challenges surrounding the defined open problems.

|
Scooped by
?
Today, 11:01 AM
|
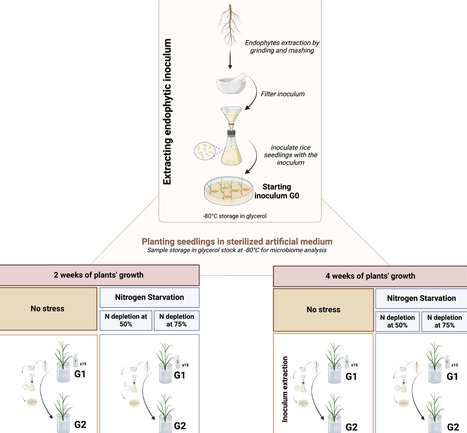
Microorganisms live in close association with plants, forming ecological interaction webs and providing beneficial traits such as nutrition, growth, and tolerance to biotic and abiotic stresses. Via the rhizosphere, plants recruit bacteria which colonize internal plant tissues, creating a spatial gradient between the rhizosphere and the endosphere. This study presents a high throughput in planta endophyte enrichment scheme designed for the identification of 'super'-endophytic bacteria which can serially colonise the rice root endosphere. Oryza sativa (rice) plants were grown in bulk soil, and endophytes were then recovered from roots. The recovered endophyte mixture was used as inoculum for the first generation of rice plantlets, which were then grown under no stress or nitrogen (N) depletion. The total endophytic community was then purified and used as a second inoculum for a new set of plants; this procedure was repeated for four generations. Enrichment patterns of root bacterial endophytes were observed, such as Kosakonia in the non-stressed plants and Ferrovibrio in plants grown under nitrogen starvation. This enrichment method proved to be suitable for the identification of endophytes which can efficiently colonise the root endosphere.

|
Scooped by
?
Today, 10:50 AM
|
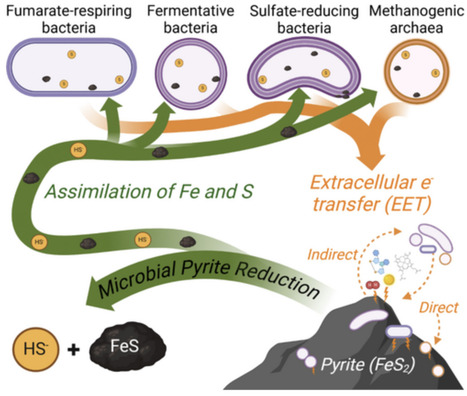
Pyrite, the most abundant iron sulfide mineral in the Earth's crust, has traditionally been considered as a sink for iron and sulfur in the absence of oxygen. Recent research, however, has shown that anaerobic methanogenic archaea can reductively dissolve pyrite and assimilate its products as sources of iron and sulfur. This study explores whether other anaerobic bacteria, including fermentative, nitrate-, iron oxide-, fumarate-, and sulfate-respiring bacteria, can also reduce pyrite and use its dissolution products as sources of iron and sulfur. Results indicate that heterotrophic bacteria respiring fumarate or sulfate, or fermenting organic carbon, can reduce pyrite and assimilate released iron and sulfur. In contrast, nitrate- or iron oxide-respiring cells did not reduce pyrite, suggesting that microbial pyrite reduction is metabolism-specific. All strains capable of reducing pyrite could also use mackinawite as an iron and sulfur source. With the exception of fermentative Bacteroides, strains did not require direct contact with pyrite to reduce the mineral, indicating extracellular electron transfer via electron shuttles. These findings expand the known diversity of microbial groups capable of pyrite reduction and highlight the mineral's lability in various anaerobic environments, with potential implications for the biogeochemical cycles of iron, sulfur, carbon, and oxygen.

|
Scooped by
?
Today, 10:25 AM
|
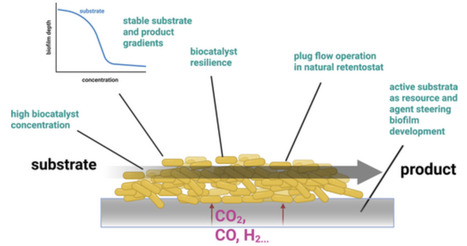
Biofilm-based production systems offer enhanced robustness, higher biomass densities and improved genetic stability compared to traditional stirred tank reactors, presenting promising alternatives for sustainable, efficient biotechnological manufacturing despite challenges in reactor design and process control.

|
Scooped by
?
Today, 10:18 AM
|
Phenazines are bioactive secondary metabolites with antifungal, anticancer, and insecticidal properties, while hydroxylated derivatives often exhibit enhanced bioactivity. 2-hydroxyphenazine (2-OH-PHZ), which is synthesised by the flavin-dependent monooxygenase PhzO from phenazine-1-carboxylic acid (PCA), shows better bioactivity against the pathogenic fungus Gaeumannomyces graminis vars. tritici. However, the low catalytic efficiency (10%–20% conversion) of PhzO limited 2-OH-PHZ production. To boost PhzO activity, engineering flavin reductase (Fre)-mediated FADH2 regeneration was applied to Pseudomonas chlororaphis LX24AE. Remarkably, this approach improved catalytic efficiency from 25% to 40% and increased the production of a novel dihydroxylated derivative. Then, it was first characterised by UPLC-MS and NMR, and identified as 3,4-dihydroxyphenazine-1-carboxylic acid (3,4-OH-PCA). Next, the Fre-PhzO module through heterologous co-expression in P. putida KT2440 demonstrated a 4.5-fold enhancement in hydroxylation efficiency relative to the PhzO mono-component system, which also confirmed that PhzO and flavin reductase are essential for 3,4-OH-PCA biosynthesis. Moreover, in vitro assays further verified that PhzO exhibits FAD-dependent catalytic promiscuity, simultaneously generating 2-OH-PCA and 3,4-OH-PCA. Furthermore, in vitro and in vivo assays demonstrated that substrate concentration affected the distribution of products. Finally, cytotoxicity evaluation of the isolated 3,4-OH-PCA was performed, and it showed substantial inhibition against oesophageal cancer TE-1 cells and human cervical cancer HeLa cells with an IC50 value of 8.55 μM and 17.69 μM, respectively. This work redefines PhzO as a promiscuous biocatalyst capable of dual hydroxylation, offering a modular platform for engineering bioactive phenazine derivatives.

|
Scooped by
?
Today, 10:07 AM
|
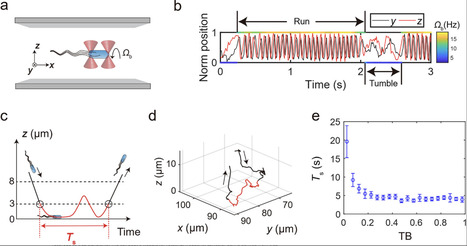
In natural environments, solid surfaces present both opportunities and challenges for bacteria. On one hand, they serve as platforms for biofilm formation, crucial for bacterial colonization and resilience in harsh conditions. On the other hand, surfaces can entrap bacteria for extended periods and force them to swim along circular trajectories, constraining their environmental exploration compared to the freedom they experience in the bulk liquid. Here, through systematic single-cell behavioral measurements, phenomenological modeling, and theoretical analysis, how bacteria strategically navigate these factors is revealed. It is observed that bacterial surface residence time decreases sharply with increasing tumble bias from zero, transitioning to a plateau at the mean tumble bias of wild-type Escherichia coli (≈0.25). Furthermore, it is found that bacterial surface diffusivity peaks near this mean tumble bias. Considering the phenotypic variation in bacterial tumble bias, which is primarily induced by noise in gene expression, this reflects a strategy for bacterial offspring persistence: In the absence of stimulus cues, some bacteria swiftly escape from the nearby surface in case it lacks nutrients, while others, with longer surface residence times, explore this 2D environment most efficiently to find potential livable sites.

|
Scooped by
?
Today, 9:33 AM
|
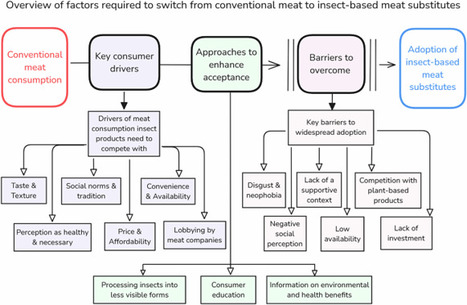
The substantial environmental footprint of meat production means that dietary shifts are needed to reduce greenhouse gas emissions. Insects may offer one alternative, but must first be widely accepted and consumed by the general public. This review evaluates the prospects of insect-based foods to compete with meat. We find that insect-based foods face major challenges, including low consumer acceptance and limited investment. They have a low likelihood of significantly reducing meat consumption, particularly when compared to more accepted plant-based alternatives.

|
Scooped by
?
Today, 8:45 AM
|
Escherichia coli, a key workhorse in industrial biotechnology, is commonly grown on glucose, which supports rapid growth but leads to acetate overflow, which instead inhibits growth, diverts carbon from the production pathway, and reduces productivity. Recent studies suggest that acetate may also have beneficial effects in glucose-grown E. coli, but its potential in bioprocesses remains unexplored. In this study, we systematically investigated acetate's impact on bioproduction using a kinetic model of glucose and acetate metabolism in E. coli. The model predicts that acetate can enhance bioproduction in glucose-grown E. coli through three mechanisms: (i) by minimizing acetate overflow, thereby reducing carbon loss, (ii) by increasing acetyl-CoA levels, thereby boosting the biosynthetic flux of acetyl-CoA-derived compounds, and (iii) by promoting biomass accumulation, thus improving overall productivity. We experimentally validated the predictions of the model for mevalonate and 3-hydroxypropionate production, where acetate supplementation increased productivity by 117% and 34%, respectively. Our findings provide a valuable framework for optimizing E. coli-based bioprocesses and highlight acetate's underutilized potential in biotechnology. By leveraging acetate from waste streams as a metabolic booster, this approach could contribute to more sustainable and environmentally friendly bioprocesses.

|
Scooped by
?
Today, 12:38 AM
|
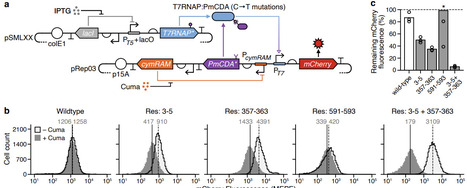
Protein-protein conjugation systems are a powerful way of creating fusion proteins and enable the dynamic combination of protein domains with diverse functionalities. However, the insertion of these systems into enzymes is often performed with little consideration of the structural impact they might have. This is particularly relevant when modifying complex molecular machines that transition between numerous conformational states. Here, we address this issue by developing SIMPLIFE, a computational workflow that supports the design of optimal insertion sites for conjugation tags based on the structure of the proteins involved and performs localised residue redesign where needed. We demonstrate how SIMPLIFE can be used to effectively augment the function of T7 RNA polymerase using the DogCatcher-DogTag system, enabling diverse and dynamically varying mutations within a targeted region of DNA. This work demonstrates the power of combining biophysical and machine learning based approaches for protein structure prediction to efficiently augment the function of molecular machines, accelerating our ability to combine complex biochemical functionalities in new ways.

|
Scooped by
?
June 22, 11:36 PM
|
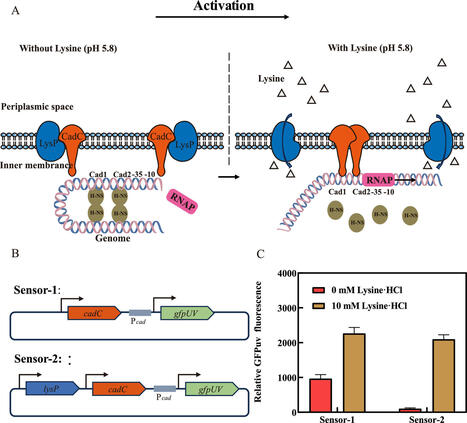
As one of the most promising monomers of biobased polyamides, cadaverine has wide industrial application prospects. However, in microbial cadaverine fermentation with glucose as the sole carbon source, the impaired coordination between precursor (lysine) utilization and cytotoxic cadaverine accumulation has been identified as the primary bottleneck limiting high-yield biosynthesis. Here, we developed a lysine biosensor in Escherichia coli to dynamically regulate cadaverine biosynthesis. Here, we developed a lysine biosensor based on the lysine transporter protein LysP, the transcription activator CadC, and the GFPuv gene under the control of the Pcad promoter. However, the engineered lysine biosensor system had a low dynamic range and a narrow pH operating range. Therefore, a multilevel optimization strategy, which included the introduction of key point mutations and engineered promoter modifications, were introduced to improve the performance of the biosensor, resulting in significant improvements in the dynamic range and lysine response. Moreover, we engineered a cadaverine-producing E. coli strain by increasing the supply of the lysine precursor, overexpressing key cadaverine synthesis genes, and knocking out genes related to metabolic bypass. The lysine biosensor was subsequently implemented to dynamically regulate cadaverine biosynthesis, resulting in a 48.10% increase in the production titre (33.19 g/L) and a 21.2% increase in cell growth compared with those resulting from the strain with constitutive expression. This is the first report in which a lysine biosensor constructed in E. coli could dynamically regulate cadaverine synthesis to improve its yield and biomass. This strategy provides new insights into the metabolic engineering of lysine and its derivatives in E. coli.

|
Scooped by
?
June 22, 10:45 PM
|
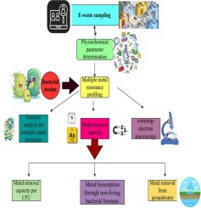
Samples were collected from two e-waste dumping sites (Mehmood Booti (31°36′28″N, 74°23′36″E) and Lakhodair (31°37′36.6″ N, 74°25′07.6″ E)) in Lahore, Pakistan. A portable multiparameter was used to determine physicochemical parameters such as temperature, pH, electrical conductivity, turbidity, total suspended particles, and total dissolved solids. Minimal salt broth was used for the determination of the minimal inhibitory concentration of the bacterium against all heavy metals. Bacterial morphology was observed under a scanning electron microscope with and without metal stress. The temperature range for all these samples was 28.7 to 35.7 °C, while the pH range was 6.7 to 7.89. The other parameters range, such as electrical conductivity µS/cm (698–8742), turbidity (14.2–103), total suspended particles (31–698), and total dissolved solids (564–23456). The lead concentration in the Mehmood Booti soil sample was 1800 mg/kg, while in the Lakhodair soil, it was 1567 mg/kg. Microbacterium sp. strain 1S1 was utilized for bioremediation assay at the lab and pilot scale. The resistance capacity of this bacterium against different metals was in the following order: As > Pb > Cd > Cu > Cr > Ni. The bioremediation potential of the bacterium against arsenic was 81.33 % and 96 % after 2 and 4 days. The least activity was observed against nickel, which was 17 and 28.33 % after 2 and 4 days. The metal removal capacity per CFU was the maximum for lead and arsenic compared to other metals, which were 1.99E-7 and 1.45E-07. The heat-inactivated bacterial cells removed arsenic in higher concentrations and lead in lower concentrations. The electron microscopy showed no significant alteration in bacterial morphology in control and metal-treated bacterial cells. The nanopore long-read sequencing analysis revealed that cadmium, nickel, copper, and arsenic resistance genes were found on the bacterial genome. No genes were found for lead and chromium but 849 hypothetical coding sequences having unknown functions were present on the bacterial genome. So, the Microbacterium sp. strain 1S1 is a potential candidate for the removal of heavy metals from e-waste dumping sites.
|
 Your new post is loading...
Your new post is loading...










acetate can be co-utilized with glycolytic substrates (Enjalbert et al., 2017; Millard et al., 2023, 2021), potentially accelerating bioprocesses by enhancing the production of acetyl-CoA-derived compounds.