Your new post is loading...
 Your new post is loading...

|
Scooped by
Judy Curtis / SIPR
October 11, 2017 2:26 PM
|
Pharmaceutical Engineering is ISPE's bi-monthly technical magazine published for Members engaged in all aspects of R&D and manufacture of safe and effective medicines covering topics important to the global pharmaceutical industry across all sectors, including traditional pharma, biotech, innovator and generics. PE presents valuable information on the latest scientific and technical developments, regulatory initiatives and innovative solutions to real-life problems and challenges through practical application articles and case studies. Technical articles will demonstrate global best practices in engineering and design; product development; technology transfer; manufacturing process development and scale-up; commercial manufacturing; quality and compliance; and product lifecycle management.

|
Scooped by
Judy Curtis / SIPR
September 9, 2017 9:15 AM
|
The new MDR and IVDR are huge departures from the historical directives they are replacing. The changes do not grandfather programs and will become law across all EU member states at the end of the transition period. Currently approved products and new submissions must consider the implications of these new regulations now during the transition period to be ready to align their programs with these new regulations.

|
Scooped by
Judy Curtis / SIPR
July 5, 2017 2:12 PM
|
Phase-Appropriate Frameworks at the Intersection of CMC and cGMP Pathways - article by Mihaela Siminiau, Ph.D, Director of Regulatory Compliance at Pharmatech Associates.

|
Scooped by
Judy Curtis / SIPR
May 11, 2017 11:05 AM
|
What’s a pharma manufacturer to do with all this hot, sizzling data?

|
Scooped by
Judy Curtis / SIPR
March 10, 2017 11:21 AM
|
The new FDA guidance is a simple one, providing best practices and high-level insight into the components of a quality agreement. T

|
Scooped by
Judy Curtis / SIPR
February 27, 2017 1:49 PM
|
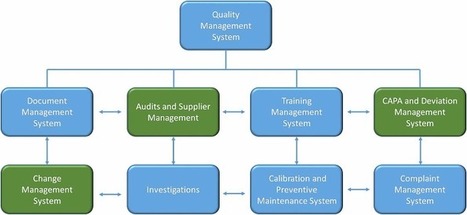
In the U.S. and Europe, the approach to ensuring drug quality has many more similarities than differences. But one key difference has always been the additional responsibilities placed upon the role of a Qualified Person or QP in ensuring the quality of a drug product. The European Union (EU) recently put in place new legislation under Annex 16, broadening the role and accountability of the QP with regard to responsibilities for batch release. The new legislation reflects the European Medicines Agency’s (EMA) efforts to introduce new quality control strategies that address today’s complex global pharmaceutical supply chain which includes new technologies such as Process Analytical technology (PAT), Real Time Release Testing (RTRT), and the growing problem of falsified medicines. This new directive has been in development since 2011 and replaces the old Annex 16 in effect since 2002. The changes are extensive and went into law in April 2016.

|
Scooped by
Judy Curtis / SIPR
January 4, 2017 1:43 PM
|
2016 Wrap-Up: Looking Ahead to 2017 and Beyond

|
Scooped by
Judy Curtis / SIPR
November 2, 2016 10:16 AM
|
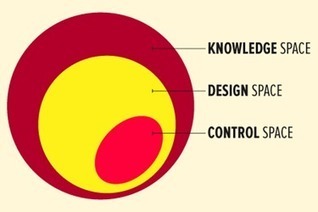
Design space is a scientific concept used in the pharmaceutical/biopharmaceutical industry to support and assure product quality.

|
Scooped by
Judy Curtis / SIPR
September 15, 2016 12:48 PM
|
Controlled Environments is a leading source of information on contamination prevention, detection, and control for cleanrooms and critical environments. Controlled Environments provides relevant and timely content on trends, technology, and applications for controlled environments professionals. Controlled Environments covers everything from pure, materials to protective packaging, from state-of-the-art facility construction through day-to-day cleaning and control challenges that affect quality and yield. The print and online Buyer's Guide provides a single-source listing of vendors, products, equipment, services, and supplies for microelectronics, pharmaceutical, and life science industries. Free subscriptions to our comprehensive daily e-newsletter and monthly magazine available at www.cemag.us.

|
Scooped by
Judy Curtis / SIPR
August 1, 2016 12:29 PM
|
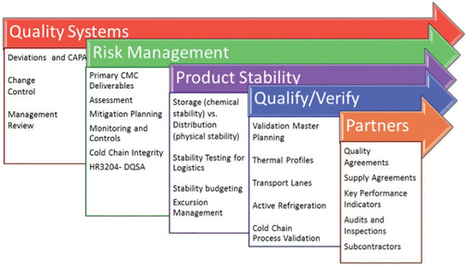
Cold chain performance is subject to the same pressures as normal supply chain process to push performance and drive down costs. Many pharma companies have turned to third party logistics (3PLs) firms that are incentivized to make the necessary investments in technology, infrastructure, and systems to drive continuous improvement and gain a competitive market edge. -- Article by Bikash Chatterjee, featured in July/ August 2016 issue of Pharmaceutical Outsourcing.

|
Scooped by
Judy Curtis / SIPR
June 8, 2016 6:43 PM
|
When we think of risk in the context of drug development and manufacturing, it is human nature to associate any risk-based approach with adding risk...instead of managing it.

|
Scooped by
Judy Curtis / SIPR
June 3, 2016 10:15 AM
|
Richard Aleman of Pharmatech Associates, profiled in Controlled Environments magazine - June 2016

|
Scooped by
Judy Curtis / SIPR
May 10, 2016 3:08 PM
|
Since the first complete human genome sequence was mapped in 2003, pharma and biotech investigators have been looking for new and innovative ways to gain insight into the genetic drivers of the
|

|
Scooped by
Judy Curtis / SIPR
September 26, 2017 4:40 PM
|
Success in developing a drug product, medical device, or drug substance requires navigating the tradeoffs and decisions of today's complex global regulatory environment.

|
Scooped by
Judy Curtis / SIPR
August 30, 2017 4:07 PM
|
"The FDA has given us the green light to assess and manage risk earlier in the drug development cycle."

|
Scooped by
Judy Curtis / SIPR
June 1, 2017 1:21 PM
|
Regulatory Forum column, by Bikash Chatterjee in May/June 2017 issue of Controlled Environments magazine.
As the deadline looms for drug master files, the FDA moves toward e-filing.

|
Scooped by
Judy Curtis / SIPR
April 13, 2017 2:27 PM
|
Integrating a contract service provider (CSP) as part of a supply chain to bring a product to market is a critical decision that impacts many aspects of bringing a pharmaceutical product to market, safely and effectively.

|
Scooped by
Judy Curtis / SIPR
February 27, 2017 2:44 PM
|
In November 2016, the FDA issued new guidance for industry titled Contract Manufacturing Arrangements for Drugs: Quality Agreements.

|
Scooped by
Judy Curtis / SIPR
February 1, 2017 12:09 PM
|
In November 2016, the FDA issued new guidance for industry titled Contract Manufacturing Arrangements for Drugs: Quality Agreements.

|
Scooped by
Judy Curtis / SIPR
December 1, 2016 3:07 PM
|
Controlled Environments is a leading source of information on contamination prevention, detection, and control for cleanrooms and critical environments. Controlled Environments provides relevant and timely content on trends, technology, and applications for controlled environments professionals. Controlled Environments covers everything from pure, materials to protective packaging, from state-of-the-art facility construction through day-to-day cleaning and control challenges that affect quality and yield. The print and online Buyer's Guide provides a single-source listing of vendors, products, equipment, services, and supplies for microelectronics, pharmaceutical, and life science industries. Free subscriptions to our comprehensive daily e-newsletter and monthly magazine available at www.cemag.us.

|
Scooped by
Judy Curtis / SIPR
September 29, 2016 5:12 PM
|
Combination product development is a complex matter governed by different areas of regulatory oversight. Part 2 of Bikash Chatterjee's article discussing QMS in combination products, the challenges and their resolution.

|
Scooped by
Judy Curtis / SIPR
August 15, 2016 12:03 PM
|
Combination products represent a remarkable opportunity to extend the potential patient population for biologics and improve the delivery of drug therapies. This is one of the most dynamic segments in the life sciences, projected to grow to $115 billion by 2019. Article by Bikash Chatterjee, Pharmatech Associates - Part 1.

|
Scooped by
Judy Curtis / SIPR
June 10, 2016 12:07 PM
|
Controlled Environments spoke with Bikash Chatterjee, the author of the CE column “Regulatory Forum.” Bikash is President and Chief Science Officer of Pharmatech Associates, a Hayward, Calif.-based company that provides consulting and services to the regulated life science industry.

|
Scooped by
Judy Curtis / SIPR
June 7, 2016 10:59 AM
|
The pharma industry is exploring the use of the Electronic Product Code Information Service to help meet the immediate and long-term requirements of the Drug Supply Chain Security Act

|
Scooped by
Judy Curtis / SIPR
May 24, 2016 8:32 PM
|
"Ransomware’s threat to healthcare," by Bikash Chatterjee, Pharmatech Associates. From laptops to smartphones to intelligent car consoles, securing proprietary or sensitive data has never been more difficult. With systems designed to promote connectivity, the threat profile today is changing as fast as the sophistication of our electronic devices. The rising tide of ransomware attacks targeting hospitals reveals a threat that goes beyond protecting data from theft, as crypto-extortion can affect data in place. Hackers already exploit inherent vulnerabilities in health IT systems and medical devices, many of which run outdated and vulnerable software.
|




 Your new post is loading...
Your new post is loading...