Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account


 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
From the INTERPHEX 2013 conference, Wai Wong, VP and GM of global operations at Pharmatech Associates, discusses the new process validation guidance.
Judy Curtis / SIPR's insight:
Legacy products pose a process validation challenge, since little historical data may be available. Risk and gap analysis and tools like FMEA offer some help in applying new guidance, says Wai Wong, VP and GM of global operations at Pharmatech Associates.
Bikash Chatterjee discusses the importance of linking process design to product performance, and defining critical process parameters.
Ever since the FDA issued its landmark guidance Pharmaceutical GMPs-A Risk Based Approach in 2004, the industry has been struggling with how to demonstrate process understanding as a basis for quality.
Judy Curtis / SIPR's insight:
Article by Bikash Chatterjee of Pharmatech Associates published in the July/August 2013 edition of Controlled Environments magazine. The new ICH Q11 guidance represents the most recent example of the FDA’s commitment to the principles of Quality by Design (QbD), intended to encourage the use of science-based and risk-based approaches of the drug manufacturing process at each stage in the lifecycle.
In January 2011 the FDA issued the New Process Validation Guidance. This modern definition of Process Validation has abandoned the concept of a one-off activity where success consists of obtaining three commercial batches of product, in favor of a scientific approach."...
Judy Curtis / SIPR's insight:
Article by Michele Levenson and Bikash Chatterjee of Pharmatech Associates, published in the September 2013 edition of Pharmaecutical Processing magazine. |
In the U.S. we are accustomed to trusting that the medications we take are real and not fake. But incidents of counterfeiting reported by drug makers have increased steadily over the last decade to more than 1,700 worldwide last year.
Judy Curtis / SIPR's insight:
Article published in the July 2013 edition of Pharmaceutical Processing magazine by Bikash Chatterjee of Pharmatech Associates. This topic was presented by Chatterjee at the INTERPHEX 2013 conference in New York City.
If we agree that quality must be the primary driver for our industry to emerge from this quagmire of negative public sentiment and regulatory action, is it possible to build a ROI justification to incentivize organizations to pursue higher quality?
Judy Curtis / SIPR's insight:
Blog post in PharmaEvolution by Bikash Chatterjee of Pharmatech Associates - August 30, 2013
Three companies, three stages of development, and one key strategy can benefit them all: risk-based validation.
Judy Curtis / SIPR's insight:
Article by Moria Feighery Ross of Pharmatech Associates, published in the August 2013 edition of Pharmaceutical Manufacturing magazine. A discussion in practical terms of how a risk-based validation system aids a company to respond to change.
CPhI Announces Members for Expert Industry Panel | Pharmaceutical Technology Magazine | The founding panel members are: Ali Afnan, president at Step Change Pharma; Brian Carlin, director of Open Innovation at FMC; Bikash Chatterjee, president and CTO of Pharmatech Associates... |


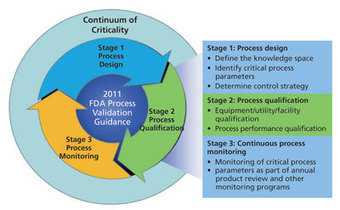










In its December 2013 edition, BioPharm International has published Part 1 of a three-part series of articles by Mark Mitchell of Pharmatech Associates: "Determining Criticality – Process Parameters and Quality Attributes." The author is breaking new ground in defining the notion of criticality with a framework that maps to the three phases of the Process Validation lifecycle.