Excerpt from 2018 CPhl Annual Report, from article by Bikash Chatterjee, President and CSO, Pharmatech Associates:
“We have seen groundbreaking drug therapies and diagnostics approved in the last five years that position regulatory bodies to embrace these new innovations. Whether risk is managed via enhanced control and oversight--such as with the EUs GDPR legislation-- or is a by-product of intelligently gathered real-world data, as provided under the US’s 21st Century Cures Act, the regulatory evaluation in each framework required to evaluate these new technologies will be grounded in today’s scientific tools and analytic techniques. Technology is playing an increasingly large role in improving rates of attrition. Over the next five years, big data will catalyse drug discovery, with R&D leading to quicker advancement of more targeted therapies” said Chatterjee.



 Your new post is loading...
Your new post is loading...






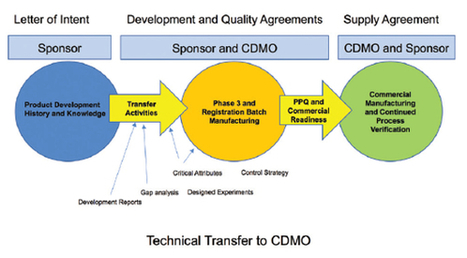









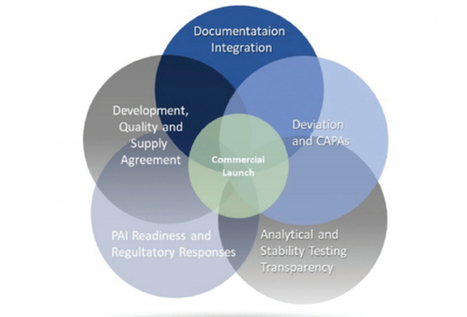
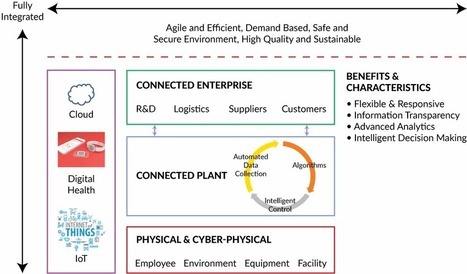





Lynn Hansen, RAC, of Pharmatech Associates writes in her latest Regulatory Forum column in Controlled Environments magazine about what is new in the new regulatory affairs certifications for 2019, and why it is important for regulatory professionals.
In summary, the new format accommodates the practical deployment of regulatory responsibilities in the industry, which is often regionally oriented with an emphasis on drugs and / or medical devices. While the RAC certification process and exams are voluntary, organizations that employ RAC certification professionals can be confident that these individuals have a firm grasp on the core components of each regulatory specialization.
RAC certified professionals can be confident that their foundational expertise is both current and correct; this often lays the groundwork to take on a more challenging role within an organization.