Pharmaceutical and Healthcare Industry books from Gower
'To know your future you must know your past.'
quotes Gower author Bikash Chatterjee in his new book Applying Lean Six Sigma in the Pharmaceutical Industry. 'The truth is, any improvement effort must understand the factors that formed its current state if an effective improvement is to be achieved.'
Wise words indeed!
There are plenty more wise words within the content of Gower's new Pharmaceutical and Healthcare Industry Catalogue. Packed with volumes from expert authors such as Ed Schoonveld, John Ansell, Lorri Zipperer and Laura Brown, it contains a wealth of insight and assistance throughout all aspects of the industry. We have also added a healthy dose of leadership, safety and risk books for good measure.
You can view the catalogue on our website in eithereCatalogue or standard PDF formats.
Each title within the catalogue is hyperlinked so you can simply click to find out more information, download a free sample chapter or order online.
Remember, all orders placed via our websitewww.gowerpublishing.com, receive an automatic 10% discount.
If you like what you read, do sign up to the Gower newsletter where you will receive information about our latest titles, exclusive rewards, promotions and access to free content.
Via
Pharma Guy

 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...


















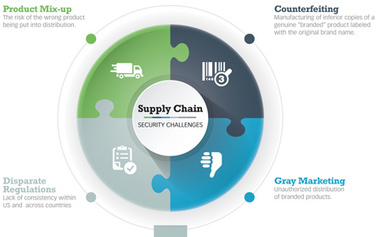




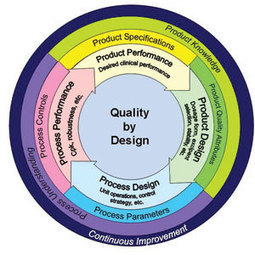






Bikash Chatterjee writes, in the latest Regulatory Forum column in Controlled Environments magazine, that realizing the promise of personalized medicine requires a shift in the way the pharma/biotech industries define quality, efficacy, and safety in personalized drug therapies as well as their companion diagnostics.
The FDA continues to refine its position on Companion Diagnostics and is working on more detailed guidance. Chatterjee warns that the agency will also have to consider the broader implications of Laboratory Derived Tests (LDTs).